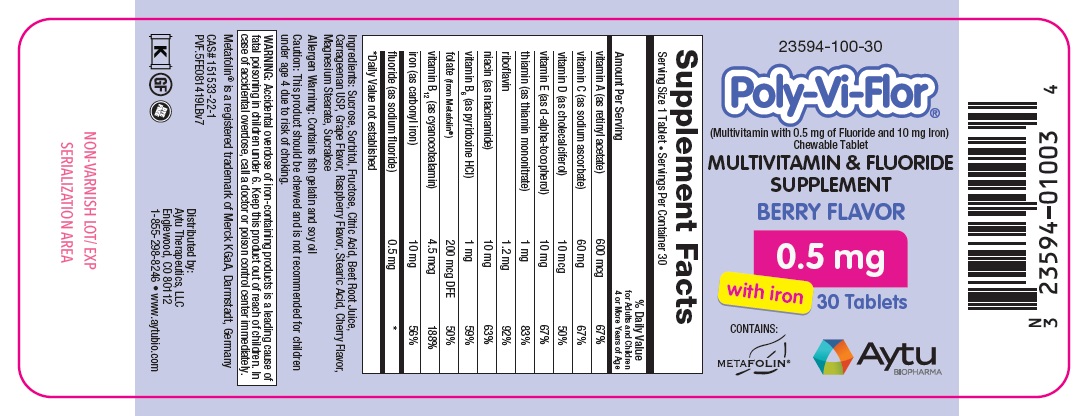
Multivitamin and Fluoride Supplement
| Supplement Facts | ||
|---|---|---|
| Serving Size 1 Tablet Servings Per Container 30 | ||
| Amount Per Serving | % Daily Value for Adults and Children 4 or More Years of Age |
|
| vitamin A (as retinyl acetate) | 600 mcg | 67% |
| vitamin C (as sodium ascorbate) | 60 mg | 67% |
| vitamin D (as cholecalciferol) | 10 mcg | 50% |
| vitamin E (as d-alpha-tocopherol) | 10 mg | 67% |
| thiamin (as thiamin mononitrate) | 1 mg | 83% |
| riboflavin | 1.2 mg | 92% |
| niacin (as niacinamide) | 10 mg | 63% |
| vitamin B6 (as pyridoxine HCl) | 1 mg | 59% |
| folate (from Metafolin®) | 200 mcg DFE | 50% |
| vitamin B12 (as cyanocobalamin) | 4.5 mcg | 188% |
| iron (as carbonyl iron) | 10 mg | 56% |
| fluoride (as sodium fluoride) | 0.5 mg | * |
*Daily Value not established
Ingredients: Sucrose, Sorbitol, Fructose, Citric Acid, Beet Root Juice, Carrageenan USP, Grape Flavor, Raspberry Flavor, Stearic Acid, Cherry Flavor, Magnesium Stearate, Sucralose
Allergen Warning: Contains fish gelatin and soy oil
Caution: This product should be chewed and is not recommended for children under age 4 due to risk of choking.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.