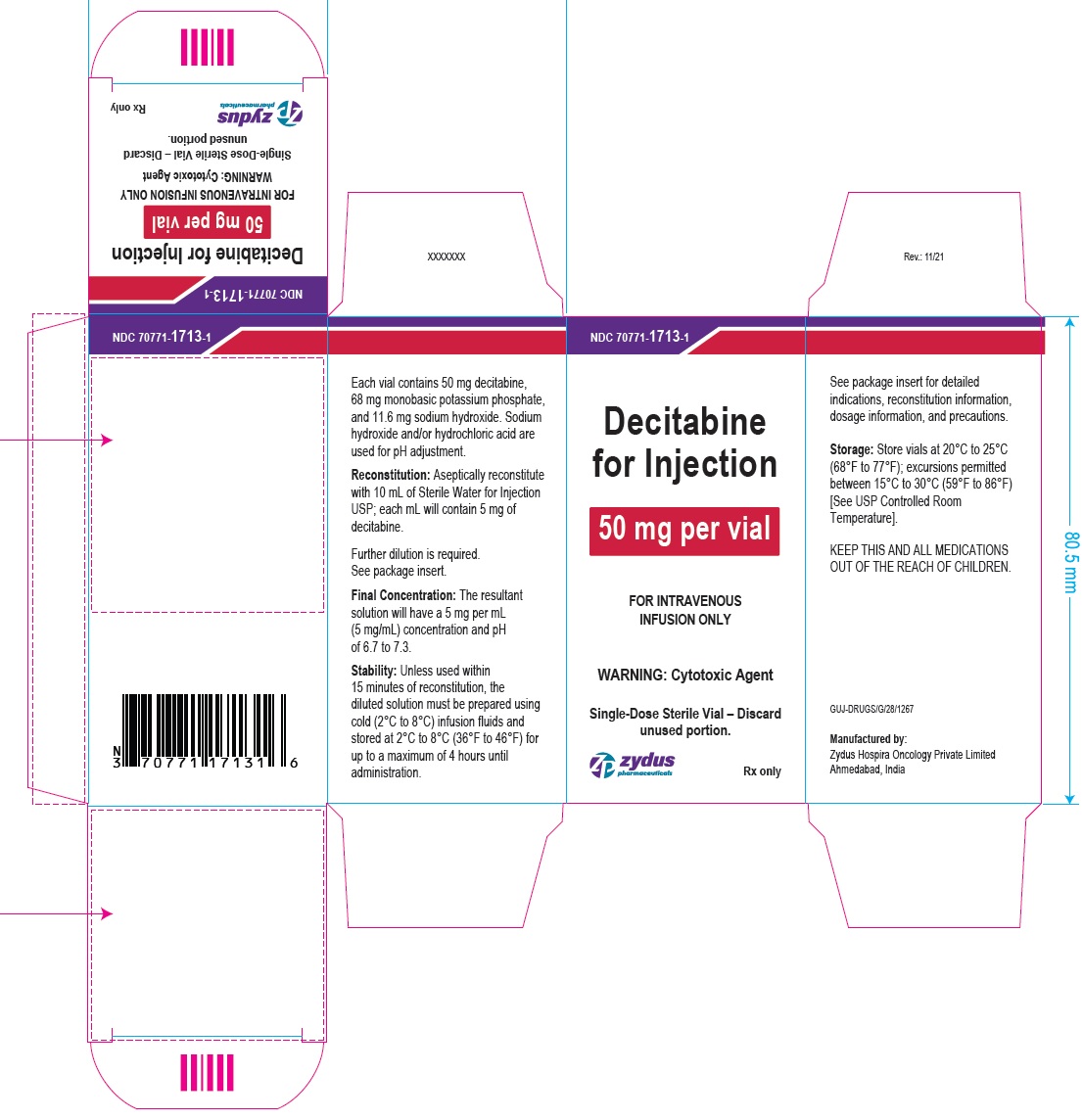
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Decitabine for Injection
50 mg per vial
FOR INTRAVENOUS INFUSION ONLY
WARNING: Cytotoxic Agent
Single-Dose Sterile Vial – Discard unused portion.
Rx only
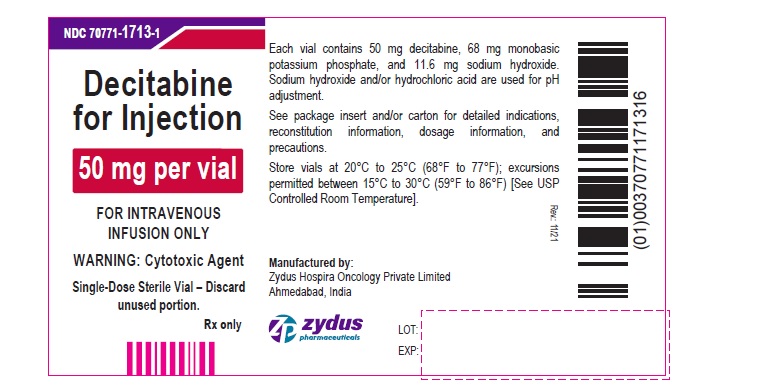
Decitabine for Injection
50 mg per vial
FOR INTRAVENOUS INFUSION ONLY
WARNING: Cytotoxic Agent
Single-Dose Sterile Vial – Discard unused portion.
Rx only