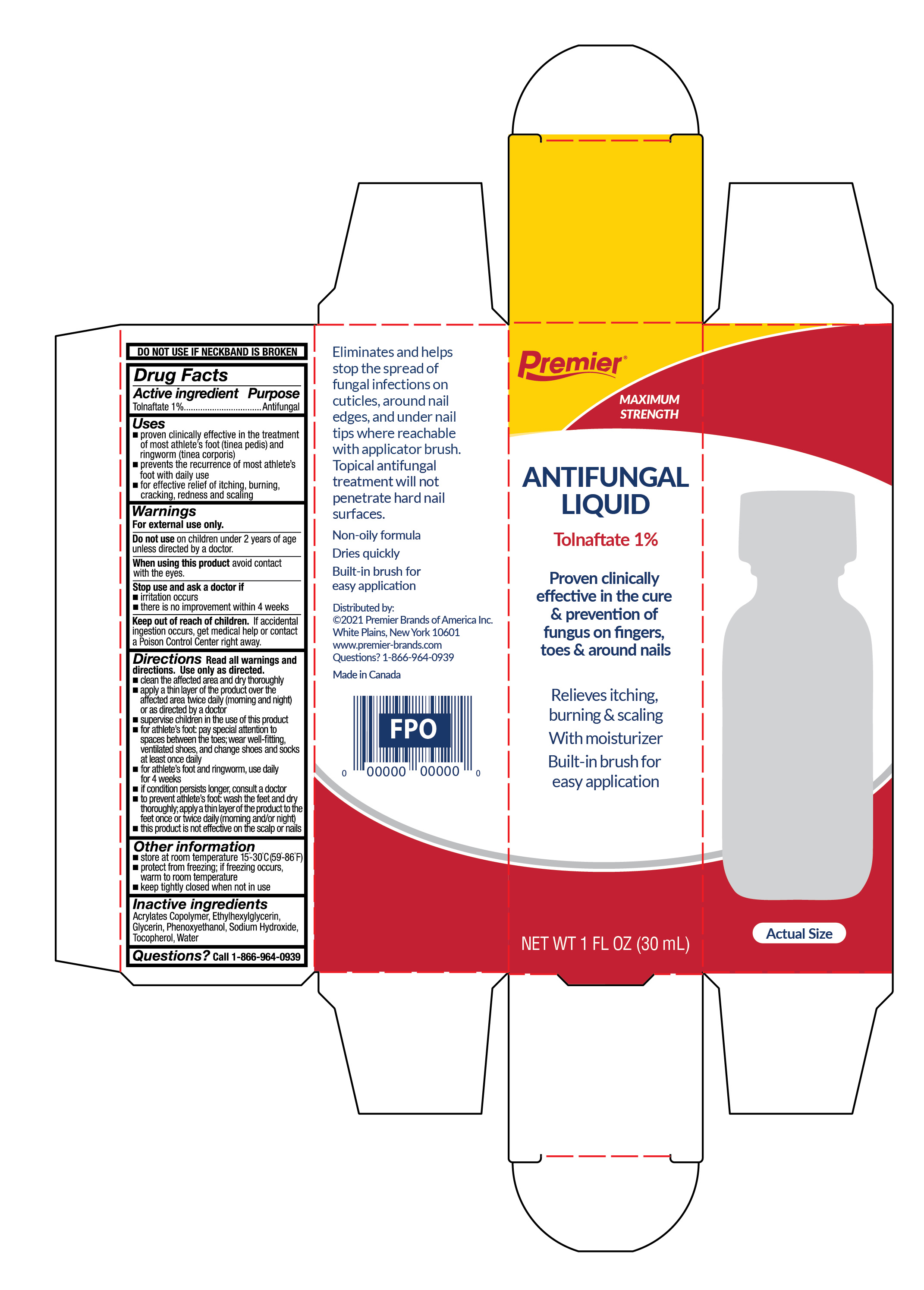
Uses
- proven clinically effective in the treatment of athlete's foot (tinea pedis) and ringworm (tinea corporis)
- prevents the recurrences of athlete's foot with daily use
- for effective relief of itching, burning, cracking, redness and scaling
Directions
Read all warnings and directions. Use only as directed.
- clean the affected area and dry thoroughly
- apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between toes
- wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks.
- If condition persists longer, consult a doctor
- to prevent athlete's foot: wash the feet thoroughly, apply a thin layer of the product to the feet once or twice daily (morning and/or night)
- this product is not effective on the scalp or nails
Other information
- store at room temperature 15°-30°C (59° - 86°F)
- protect from freezing. If freezing occurs warm to room temperature
- keep tightly closed when not in use