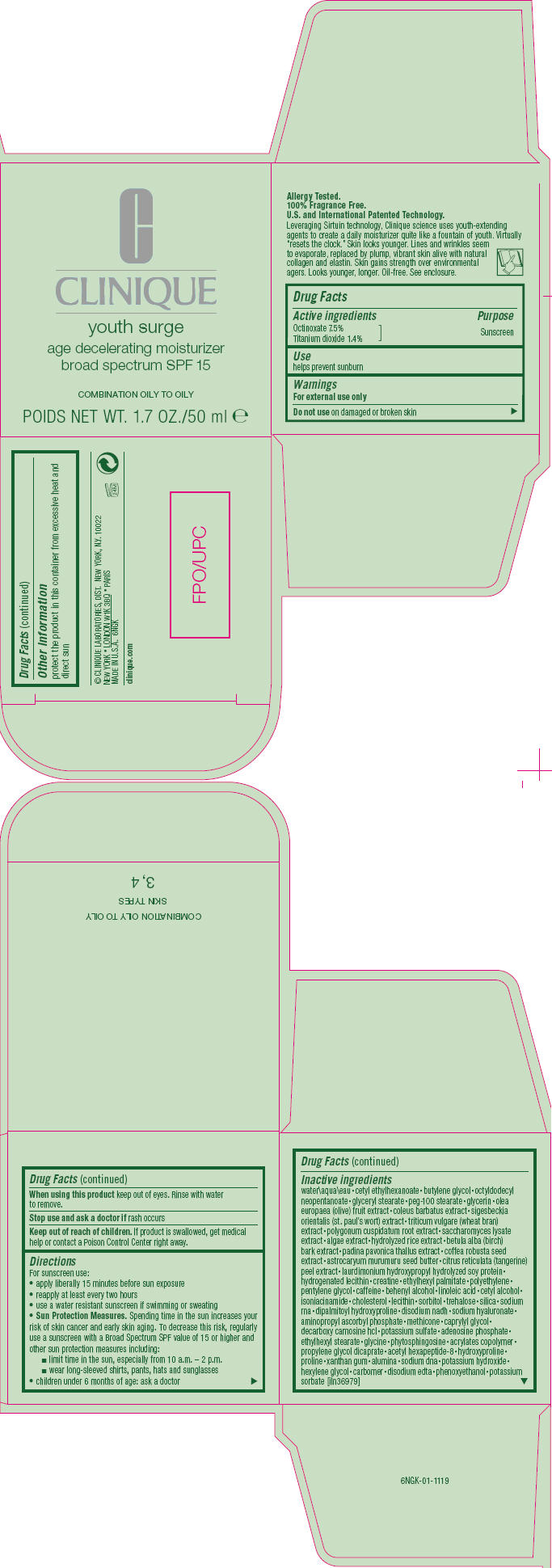
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive ingredients
water • cetyl ethylhexanoate • butylene glycol • octyldodecyl neopentanoate • glyceryl stearate • peg-100 stearate • glycerin • olea europaea (olive) fruit extract • coleus barbatus extract • sigesbeckia orientalis (st. paul's wort) extract • triticum vulgare (wheat bran) extract • polygonum cuspidatum root extract • saccharomyces lysate extract • algae extract • hydrolyzed rice extract • betula alba (birch) bark extract • padina pavonica thallus extract • coffea robusta seed extract • astrocaryum murumuru seed butter • citrus reticulata (tangerine) peel extract • laurdimonium hydroxypropyl hydrolyzed soy protein • hydrogenated lecithin • creatine • ethylhexyl palmitate • polyethylene • pentylene glycol • caffeine • behenyl alcohol • linoleic acid • cetyl alcohol • isoniacinamide • cholesterol • lecithin • sorbitol • trehalose • silica • sodium rna • dipalmitoyl hydroxyproline • disodium nadh • sodium hyaluronate • aminopropyl ascorbyl phosphate • methicone • caprylyl glycol • decarboxy carnosine hcl • potassium sulfate • adenosine phosphate • ethylhexyl stearate • glycine • phytosphingosine • acrylates copolymer • propylene glycol dicaprate • acetyl hexapeptide-8 • hydroxyproline • proline • xanthan gum • alumina • sodium dna • potassium hydroxide • hexylene glycol • carbomer • disodium edta • phenoxyethanol • potassium sorbate [iln36979]