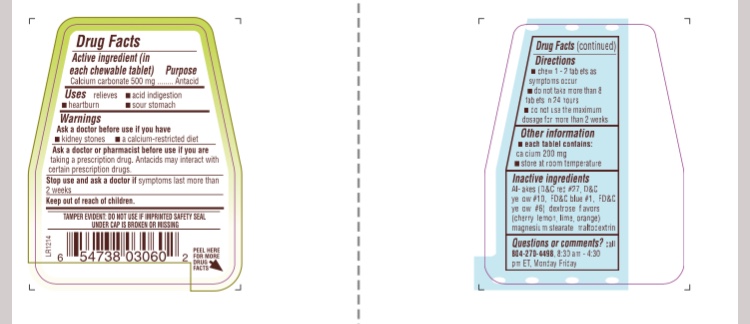
Warnings
Ask a doctor before use if you have
- kidney stones
- a calcium-restricted diet
ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- chew 1-2 tablets as symptoms occurs.
- do not take more than 8 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
Other Information
- each tablet contains: calcium 200 mg
- store at room temperature 15-30 °C (59-86 °F)
Inactive Ingredients
Al-lakes (D&C red #27, D&C yellow # 10, FD&C blue #1, FD&C yellow # 6), dextrose, flavors (cherry, lemon, lime, orange), magnesium stearate, maltodextrin