DORMOSEDAN® GEL
(detomidine hydrochloride)
Alpha2-agonist oromucosal gel
Rx only
For Sedation and Restraint in Horses Only
DESCRIPTION
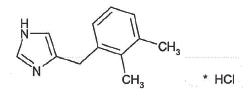
DORMOSEDAN (detomidine hydrochloride) GEL is a synthetic alpha2-adrenoreceptor agonist with sedative properties. Each mL of DORMOSEDAN GEL contains 7.6 mg detomidine hydrochloride. The chemical name is 1H imidazole, 4-[(2,3-dimethylphenyl) methyl]-hydrochloride. Detomidine hydrochloride is a white, crystalline, water-soluble substance having a molecular weight of 222.7. The molecular formula is C12H14N2•HCl and the structural formula is
DOSAGE AND ADMINISTRATION
DORMOSEDAN GEL produces sedation when administered sublingually at 0.018 mg/lb (0.040 mg/kg). DORMOSEDAN GEL must be placed beneath the tongue of the horse and is not meant to be swallowed. The dosing syringe delivers the product in 0.25 mL increments. The following dosing table may be used to determine the correct dose of DORMOSEDAN GEL (Table 1).
| Approximate body weight (lb) | Range of doses (mg/lb) | Approximate body weight (kg) | Range of doses (mg/kg) | Dose volume (mL) |
|---|---|---|---|---|
| 330 - 439 | 0.023 – 0.017 | 150 - 199 | 0.051 – 0.038 | 1.00 |
| 440 - 549 | 0.022 – 0.017 | 200 - 249 | 0.047 – 0-038 | 1.25 |
| 550 – 659 | 0.021 – 0.017 | 250 – 299 | 0.046 – 0.038 | 1.50 |
| 660 - 769 | 0.020 – 0.017 | 300 – 349 | 0.044 – 0.038 | 1.75 |
| 770 - 879 | 0.019 – 0.017 | 350 – 399 | 0.043 – 0.038 | 2.00 |
| 880 - 989 | 0.019 – 0.017 | 400 – 449 | 0.043 – 0.038 | 2.25 |
| 990 - 1099 | 0.019 – 0.017 | 450 – 499 | 0.042 – 0.038 | 2.50 |
| 1100 - 1209 | 0.019 – 0.017 | 500 – 549 | 0.042 – 0.038 | 2.75 |
| 1210 - 1320 | 0.019 – 0.017 | 550 - 600 | 0.041 – 0.038 | 3.00 |
Use impermeable gloves when handling the product. Remove the syringe from the outer carton. While holding the plunger, turn the ring-stop on the plunger until the ring is able to slide freely up and down the plunger. Position the ring in such a way that the side nearest the barrel is at the desired volume marking. Turn the ring to secure it in place. Make sure that the horse's mouth contains no feed. Remove the cap from the tip of the syringe and save for cap replacement. Insert the syringe tip into the horse's mouth from the side of the mouth, placing the syringe tip beneath the tongue at the level of the commisure of the mouth. Depress the plunger until the ring-stop contacts the barrel, depositing the product beneath the tongue.
The following picture demonstrates correct administration of DORMOSEDAN GEL beneath the tongue.
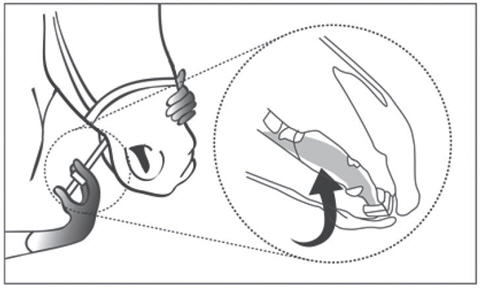
Take the syringe out of the horse's mouth, recap the syringe and return it to the outer carton for disposal. Remove gloves for disposal.
For the best results, allow adequate time (a minimum of 40 minutes) between administration of DORMOSEDAN GEL and beginning the procedure. In general, horses show sedative effects lasting approximately 90-180 minutes.
Withhold food and water until the sedative effects of the product wear off.
CONTRAINDICATIONS
DORMOSEDAN GEL is contraindicated in horses with known hypersensitivity to detomidine. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses as potentially fatal dysrhythmias may occur.
Do not use DORMOSEDAN GEL in horses with pre-existing atrioventricular (AV) or sino-atrial (SA) blocks, respiratory disease, or chronic renal failure.
WARNINGS
For sublingual use in horses only. Do not use in horses intended for human consumption.
HUMAN WARNINGS
Not for human use. Keep out of the reach of children. Use impermeable gloves during drug administration and during procedures that require contact with the horse's mouth. Following sublingual administration of detomidine oromucosal gel, drug concentrations up to 0.072 mg/mL were measured at 30 minutes post dose in equine saliva, equivalent to less than one percent of the original detomidine concentration in the gel. Mean drug concentrations fall to less than 0.010 mg/mL by 2 hours after drug administration, after which a slow decline occurs for several additional hours.
DORMOSEDAN GEL can be absorbed following direct exposure to skin, eyes, or mouth, and may cause irritation. Skin and mucosal contact with the product should be avoided. Use impermeable gloves at all times.
In case of accidental eye exposure, rinse abundantly with fresh water. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing.
Appropriate precautions should be taken while handling and using gel syringes. Accidental exposure could cause adverse reactions, including sedation, hypotension, and bradycardia. Seek medical attention immediately but do not drive because sedation or changes in blood pressure may occur.
Individuals with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid exposure to this product.
Caution should be exercised when handling sedated horses. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
Rare cases of human abuse of detomidine products have been reported. DORMOSEDAN GEL should be managed to prevent the risk of diversion, through such measures as restriction of access and the use of drug accountability procedures appropriate to the clinical setting.
Note to physician: This product contains an alpha2-adrenoceptor agonist.
PRECAUTIONS
DORMOSEDAN GEL must be placed beneath the tongue of the horse. Unlike most oral veterinary products, this product is not meant to be swallowed. Swallowing could result in ineffectiveness.
DORMOSEDAN GEL does not provide analgesia. Do not use for painful procedures.
Do not use with other sedative drugs because the effects may be additive.
Repeat dosing has not been evaluated.
The use of an alpha2-agonist reversal agent with DORMOSEDAN GEL has not been evaluated.
Before initiating any procedure, allow sedation to fully develop. Nervous or excited horses with high levels of endogenous catecholamines may exhibit a reduced pharmacological response to alpha2-adrenoceptor agonists like detomidine. In agitated horses, the onset of sedative effects could be slowed, or the depth and duration of effects could be diminished or nonexistent. When the product is administered, the animal should be allowed to rest in a quiet place for a minimum of 40 minutes.
Do not use DORMOSEDAN GEL in horses with cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation, or stress due to extreme heat, cold, fatigue, or high altitude. Protect treated horses from temperature extremes. As with all alpha2-adrenoceptor agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation), exists.
DORMOSEDAN GEL has not been evaluated in ponies, miniature horses, or horses younger than one year of age.
DORMOSEDAN GEL has not been evaluated for use in breeding, pregnant, or lactating horses.
ADVERSE REACTIONS
Clinical field study
In a US field study of 270 horses sedated to facilitate completion of various veterinary and husbandry procedures, the following adverse reactions were reported in 202 horses treated with DORMOSEDAN GEL and 68 horses treated with placebo:
| Clinical Sign | DORMOSEDAN GEL N = 202 | Placebo N =68 |
|---|---|---|
| Sweating | 20 | 0 |
| Penile relaxation | 12 | 0 |
| Bradycardia (≤ 20 bpm) | 11 | 0 |
| Second degree AV block | 9 | 0 |
| Frequent urination | 9 | 0 |
| Piloerection | 4 | 0 |
| Marked ataxia | 3 | 0 |
| Facial/oral edema | 3 | 0 |
| Hypersalivation | 2 | 0 |
| Nasal discharge | 2 | 0 |
| Flatulence | 1 | 0 |
| Muscle tremors | 1 | 1 |
| Epiphora | 1 | 0 |
| Pale mucous membranes | 1 | 0 |
| Swollen sheath | 1 | 0 |
In a laboratory study, transient erythema of the mucous membranes was seen in 2 (of 8) horses that received the recommended dose of detomidine gel.
Mild ataxia (horse stable but swaying slightly) was observed in 54% of DORMOSEDAN GEL-treated horses and in 4% of the placebotreated horses at 40 minutes post treatment administration. Moderate ataxia was observed in 25% of DORMOSEDAN GEL-treated horses (0% placebo) at 40 minutes post treatment. Moderate to marked ataxia continued to 90 minutes for 5% and to 120 minutes for 4% of DORMOSEDAN GEL-treated horses.
CONTACT INFORMATION
For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
CLINICAL PHARMACOLOGY
Detomidine is a potent non-narcotic alpha2-adrenoceptor agonist which produces sedation with a central effect inhibiting the transmission of noradrenalin-mediated nervous impulses. Blood pressure is initially increased due to peripheral vasoconstriction, subsequently dropping to normal or slightly below normal levels. Vasoconstriction may cause mucous membranes to appear pale or mildly cyanotic. This initial vasopressor response is accompanied by a compensatory marked decrease in heart rate mediated by a vagal baroreceptor. The peripheral pulse may feel weak and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by first and second degree atrioventricular blocks. Other arrhythmias may occur. Detomidine also decreases the respiratory rate and decreases body temperature. Detomidine causes depression of gastrointestinal motility due to decrease in smooth muscle activity, increases blood glucose levels due to inhibition of insulin release, and increases production of urine 2 to 4 hours after treatment. In some horses, sweating, salivation and slight muscle tremors may be seen. Partial, transient penis prolapse may occur in stallions and geldings. Because of continued lowering of the head during sedation, mucus discharges from the nose with occasional swelling of the head, particularly around the eyes, may be seen.
Detomidine is oxidized mainly in the liver. Most metabolites are excreted in the urine. Halflife (T½) is 1-2 hours. Detomidine is rapidly distributed; volume of distribution (Vd) varies between 0.69 L/kg and 1.89 L/kg. Protein binding is about 85%.
Detomidine is a high extraction ratio drug. Alterations in liver blood flow (the site of detomidine metabolism) can change the rate of drug clearance and, consequently, drug exposure. The sedative effects of detomidine (using head droop as a marker for sedation) are highly correlated to blood concentration, regardless of the route of administration.
First pass effect results in a very small portion of drug reaching the systemic circulation if it is swallowed. Sedation achieved with the DORMOSEDAN GEL is attributable to sublingual drug absorption. Peak concentrations occur approximately 1.83 hours after sublingual administration of DORMOSEDAN GEL. The peak concentrations observed after administration of DORMOSEDAN GEL are approximately 40% of those observed after intramuscular injection of detomidine solution. The absolute bioavailability of detomidine in DORMOSEDAN GEL is 22%.
EFFECTIVENESS
A prospective, randomized, masked, multi-center study was conducted to evaluate under field conditions, whether DORMOSEDAN GEL provided sufficient sedation and restraint in horses to successfully conduct procedures requiring administration of a sedative. Two hundred and seventy client-owned horses of any breed or sex were sedated to facilitate grooming (including cleaning of the prepuce), hoof care, floating teeth (manually), passage of a nasogastric tube or endoscope, or radiography. Horses were enrolled in the study if they were a yearling or older, in satisfactory body condition, and had a history of requiring sedation or other means of strong restraint to enable similar procedures to be carried out. Horses were randomly assigned to receive DORMOSEDAN GEL sublingually at 0.040 mg/ kg or placebo gel. After administration of treatment, each horse's level of sedation, degree of ataxia, heart rate and rhythm, and respiratory rate were assessed and measured to recovery. After an appropriate period of time elapsed to allow sedation to develop, a study veterinarian assessed and scored the ability to attempt and to complete the veterinary or husbandry procedure.
One hundred and twenty-nine DORMOSEDAN GEL-treated and 42 placebo-treated horses were included in the statistical analysis of effectiveness. Ninety-nine horses were excluded from the analysis due to failure to meet inclusion criteria or due to major protocol deviations. The veterinary or husbandry procedure was successfully completed for 98 of 129 DORMOSEDAN GEL-treated horses (76%) but only 3 of 42 placebo-treated horses (7%) (Table 3). The difference between the two treatments was statistically significant (p=0.0005).
| Ability to perform the procedure score* | DORMOSEDAN GEL N=129 | Placebo N=42 |
|---|---|---|
|
||
| 0 | 16 | 38 |
| 1 | 15 | 1 |
| 2 | 44 | 2 |
| 3 | 54 | 1 |
| Success (score 2 or 3) | 98 | 3 |
* 0: Poor – Strong resistance. 1: Fair. Moderate resistance. 2: Good.Some resistance, but the procedure could be performed. 3: Excellent. Procedure could be easily performed with insignificant resistance
The following success rates with DORMOSEDAN GEL were recorded for electric clipping of hair (48%), cleaning the prepuce (81%), manual floating of teeth (89%), hoof trimming or shoeing (86%), passage of a nasogastric tube or endoscope (80%), or radiography (74%). At 40 minutes post dosing, 94% of DORMOSEDAN GEL-treated horses showed minimal, moderate or marked sedation compared with 14% of the horses treated with placebo. All DORMOSEDAN GEL-treated horses had recovered from sedation by 240 minutes post treatment.
DORMOSEDAN GEL was correctly administered sublingually (beneath the tongue) in 97% of horses with mild or no objection.
ANIMAL SAFETY
In a multiple dose target animal safety study, DORMOSEDAN GEL was administered on three consecutive days to 6 horses per treatment group at 0, 1, 3 and 5 times the recommended label dose of 0.040 mg/kg.
The recommended dose (1X) induced sedation. Head droop caused transient edema of the head area, nasal/ocular discharge, and congestion of oral mucous membranes. Ataxia, sweating, and reversible penile prolapse were observed. Erythematous mucous membranes were seen at the area of dose application in 2/6 horses. Transient reductions were seen in heart rate, respiratory rate, and gut motility. Electrocardiography revealed increased incidences of vagally mediated arrhythmias (sinus arrhythmia, sinus block, 1st and 2nd degree atrioventricular block) as well as atrial or ventricular premature beats in the majority of horses. No clinical abnormalities were associated with the transient arrhythmias. Excessive or erratic urination were seen in isolated cases.
Similar treatment related findings were seen in horses receiving 3X and 5X doses. In most cases the incidence, severity, and duration of the findings were dose dependent. All findings in all dose groups were representative of the alpha2-adrenoreceptor drugs used in horses.
STORAGE INFORMATION
Store at controlled room temperature 20-25°C (68-77°F), with excursions permitted to 15-30°C (59-86°F), in the original package.
DORMOSEDAN® is a trademark of Orion Corporation.
Approved by FDA under NADA # 141-306

Mfd by:
Orion Corporation
Turku, Finland
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
Revised: March 2022
CLIENT INFORMATION SHEET FOR OWNER/HANDLER USE AND SAFETY
This summary contains important information about Dormosedan (detomidine hydrochloride) Gel. You should read this information before you administer Dormosedan Gel to your horse. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or if you want to know more about Dormosedan Gel.
What is Dormosedan Gel?
Dormosedan Gel is an oromucosal sedative containing detomidine hydrochloride. It is prescribed by veterinarians to allow procedures to be done in an anxious horse. Dormosedan Gel has not been shown to provide analgesia and should not be used for painful procedures.
How should the product be handled?
Always wear impermeable gloves when handling the dosing syringe with detomidine hydrochloride gel. Ask the veterinarian whether the gloves you plan to use are impermeable. For a minimum of 2 hours after administration, wear impermeable gloves when performing any tasks that require contact with the horse's mouth.
If you have or have had a history of cardiovascular disease (for example, hypertension or heart attack) take special precautions and avoid direct exposure to the dosing syringe. Do not come in contact with the mouth or any saliva of any horse that was treated with detomidine gel for a minimum of 2 hours.
What if I get the gel in my eyes or mouth?
Detomidine hydrochloride can be absorbed into your body after direct exposure through the eyes or mouth, and may cause irritation to these areas. In case of accidental eye exposure, flush with water for 15 minutes. If detomidine is exposed to the mucous membranes of the mouth, rinse without swallowing. In all cases of accidental exposure and possible ingestion, seek medical attention immediately. Accidental exposure could result in the drug affecting you, causing symptoms that include sleepiness, low blood pressure, and slower heart rate. DO NOT DRIVE because detomidine may cause you to feel drowsy or sleepy. Share the package information with your physician and tell the physician that the product contains an alpha2-adrenoceptor agonist.
What if I get the gel on my skin?
Detomidine hydrochloride can be absorbed into your body after direct exposure through the skin. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Contact your physician if you have any questions or concerns.
Contact Information
For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
How is Dormosedan Gel administered?
Dormosedan Gel should be given according to your veterinarian's instructions. Your veterinarian will tell you what amount of gel you should give to your horse. The appropriate dose is delivered beneath the tongue (sublingually) and is not meant to be swallowed. Make sure there is no food in the horse's mouth prior to administration.
The following drawing demonstrates correct administration of Dormosedan Gel beneath the tongue.
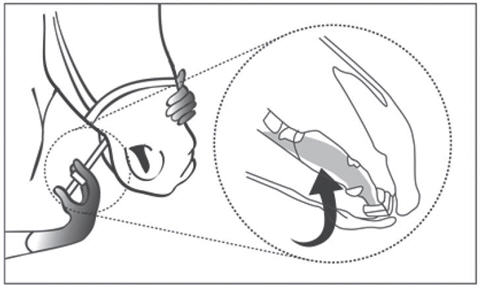
Following appropriate dosing of the gel, your horse should be kept in a quiet area until sedation is achieved.
If after 40 minutes there is inadequate sedation and you suspect that the horse swallowed or spit out some of the gel, contact your prescribing veterinarian. Do not repeat the dose.
If you believe the correct dose of detomidine gel was administered but the horse remains inadequately sedated, contact the prescribing veterinarian. Do not repeat the dose.
Contact your prescribing veterinarian immediately if the dosing syringe fails during the administration of detomidine gel and you are unsure if too much or too little of the dose was given.
Do not re-use partial dosing syringes. Any unused product or waste material should be disposed of in accordance with local requirements and Federal prescription drug disposal guidelines. Ask your veterinarian for this information.
What should I expect after administering Dormosedan Gel?
Following appropriate dosing of the gel, your horse should be kept in a quiet area. As the drug takes effect, you will typically see the head lower and the front legs plant in a firm stance. This will usually take about 40 minutes. You may also notice slight swaying, sweating, salivation and slight muscle tremors. Be careful when handling sedated horses. Handling or any other sudden stimuli, including noise, may cause a defense reaction (for example, kicking) even in a horse that appears to be fully sedated. It may take up to 3-4 hours for the horse to recover from sedation. Withhold food and water until the horse has recovered.
What else should I know about Dormosedan Gel?
As with all prescribed medicines, Dormosedan Gel should only be given to the horse for which it was prescribed. This sheet provides a summary of information about Dormosedan Gel. If you have any questions or concerns about Dormosedan Gel or its effects on your horse or yourself, talk to your veterinarian.
Revised: March 2022
