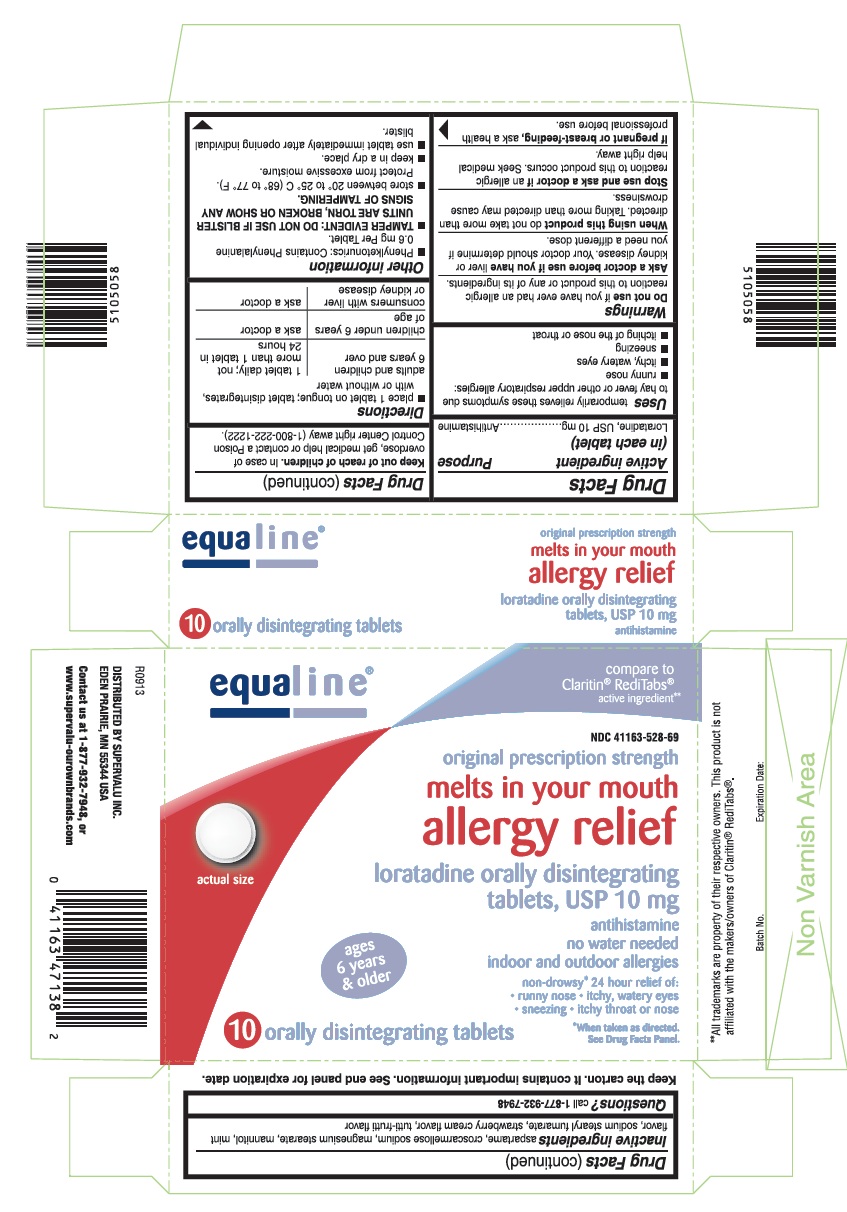
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
DIRECTIONS
- place 1 tablet on tongue; tablet disintegrates, with or without water
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
OTHER INFORMATION
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- keep in a dry place.
- use tablet immediately after opening individual blister.
INACTIVE INGREDIENTS
Aspartame, croscarmellose sodium, magnesium stearate, mannitol, mint flavor, sodium stearyl fumarate, strawberry cream flavor, tutti-frutti flavor
PRINCIPAL DISPLAY PANEL
compare to Claritin®RediTabs®active ingredient**
original prescription strength
loratadine orally disintegrating tablets, USP 10 mg
non-drowsy* 24 hour relief of:
- runny nose
- itchy, watery eyes
- sneezing
- itchy throat or nose
10 orally disintegrating tablets