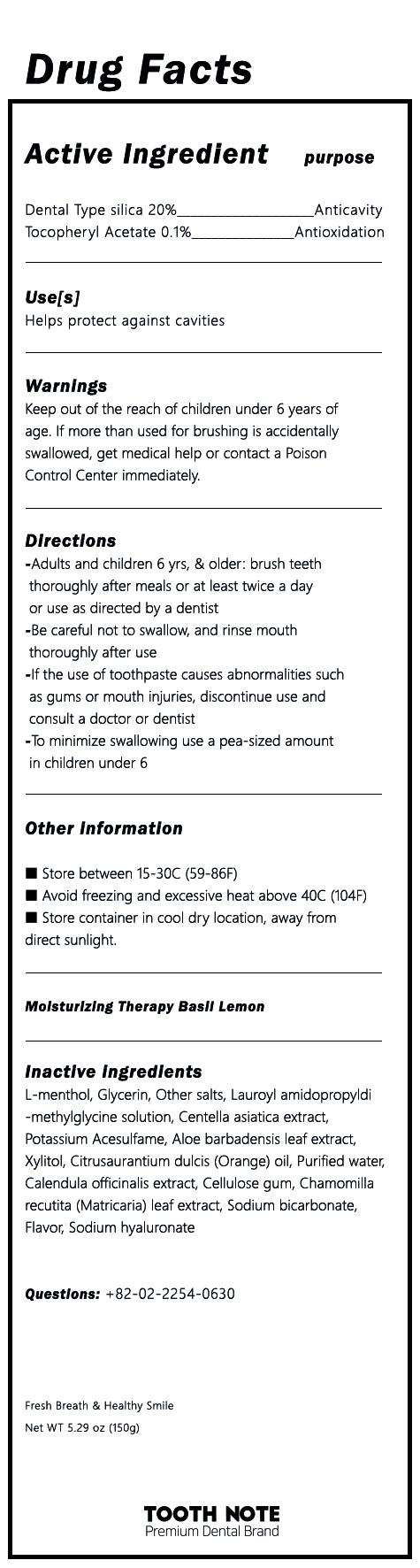
L-menthol, Glycerin, Other Salts, Lauroyl Amidopropyldi-methylglycine solution, Centella asiatica extract, Potassium Acesulfame, Aloe barbadensis leaf extract, Xylitol, Citrusaurantium dulcis (Orange) oil, Purified water, Calendula officinalis extract, Cellulose gum, Chamomilla recutita (Matricaria) leaf extract, Sodium bicarbonate, Flavor, Sodium hyaluronate
1) Be careful not to swallow, and rinse mouth thoroughly after use
2) If the use of toothpaste causes abnormalities such as gums or mouth injuries, discontinue use and consult a doctor or dentist
3) If you are a child under 6 years of age, use a small amount of toothpaste as small as pea per use, and use under the supervision of a guardian to avoid sucking or swallowing
4 If a child under 6 years of age swallowed large quantities, consult a physician or dentist immediately
5) Keep out of the reach of children under 6 years