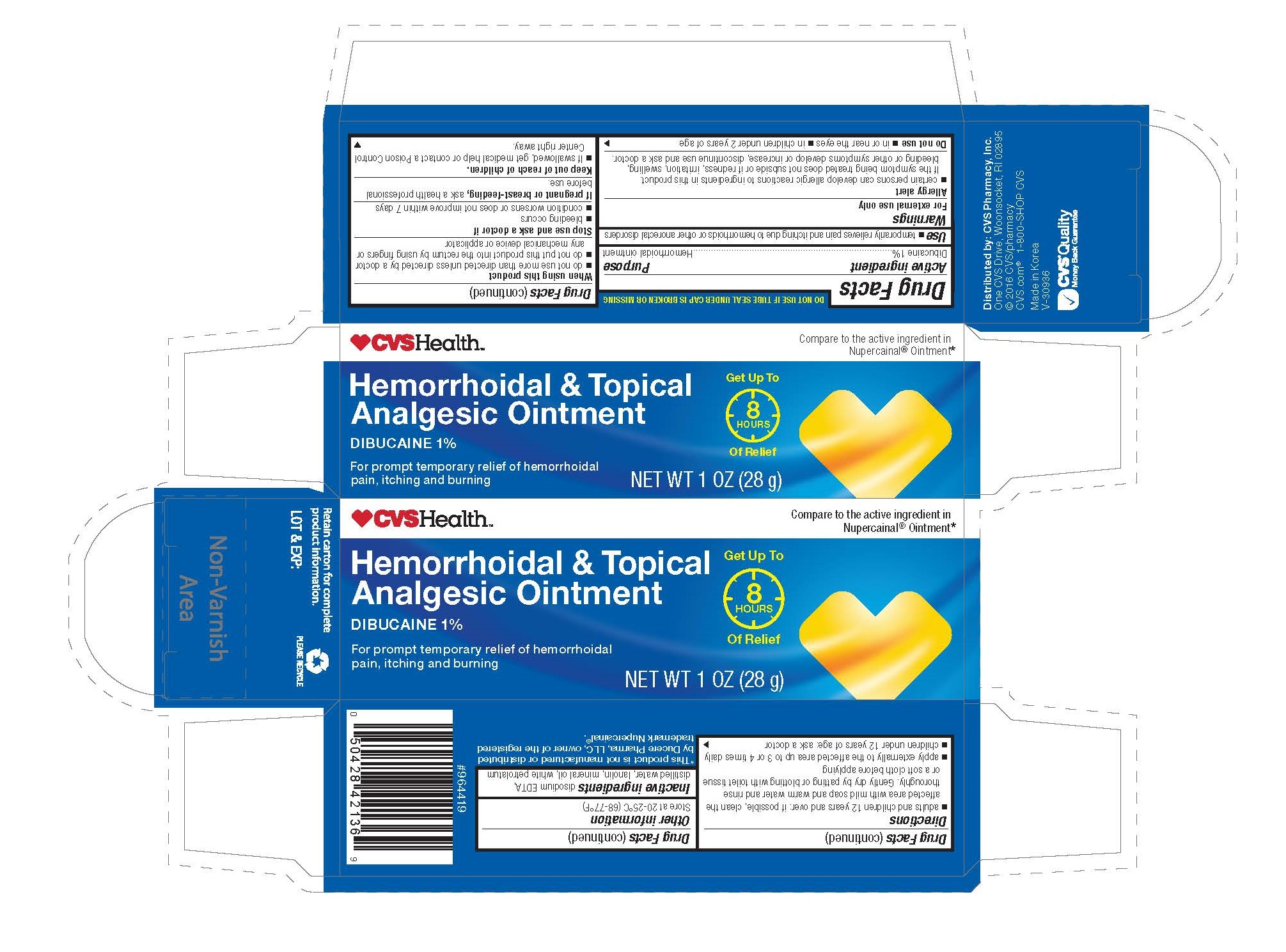
CVS HEMORRHOIDAL TOPICAL ANALGESIC- dibucaine ointment
CVS Pharmacy, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient Purpose
Dibucaine 1%...................................... Local anesthetic
Use
temporarily relives pain and itching due to hemorrhoids or other anorectal disorders
Warnings
For external use only
Allergy Alert
- Certain persons can develop allergic reactions to ingredietns in this product. If the symptoms being treated does not subside or if redness, irritation, swelling, bleeding or other symptoms develop or increase, discontinue use and ask a doctor.
Do not use
- in or near the eyes
- in children under 2 years of age
When using this product
- do not use more than directed unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- bleeding occurs
- condition worsens or does not improve within 7 days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over: if possible, clean the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply externally to the affected area up to 3 or 4 times daily
- children under 12 years of age: ask a doctor
Other information
store at 20-25°C (68-77°F)
Inactive ingredients
disodium EDTA, distilled water, lanolin, mineral oil, white petrolatum
DISTRIBUTED BY:
CVS PHARMACY, INC.
ONE CVS DRIVE
WOONSOCKET, RI 02895 USA
CVS Pharmacy, Inc.