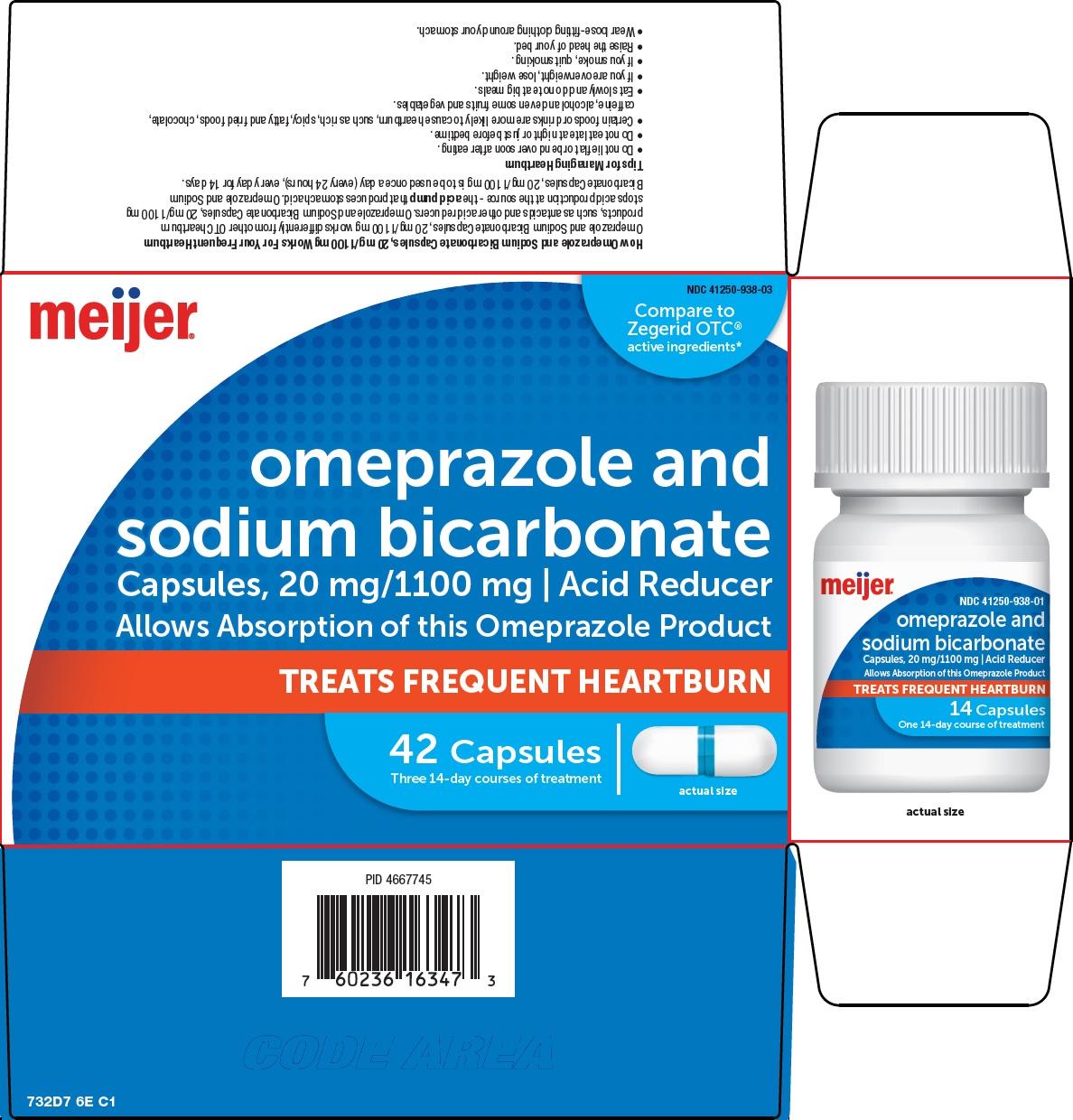
Use
- •
- treats frequent heartburn (occurs 2 or more days a week)
- •
- not intended for immediate relief of heartburn. This drug may take 1 to 4 days for full effect.
Warnings
Allergy alert: Do not use if you are allergic to omeprazole
Do not use
if you have:
- •
- trouble or pain swallowing food
- •
- vomiting with blood
- •
- bloody or black stools
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- a sodium-restricted diet
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- •
- for adults 18 years of age and older
- •
- this product is to be used once a day (every 24 hours), every day for 14 days
- •
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- •
- swallow 1 capsule with a glass of water at least 1 hour before eating in the morning
- •
- take every day for 14 days
- •
- do not take more than 1 capsule a day
- •
- do not chew or crush the capsule
- •
- do not open capsule and sprinkle on food
- •
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- •
- you may repeat a 14-day course every 4 months
- •
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- •
- children under 18 years of age: ask a doctor. Heartburn in children may sometimes be caused by a serious condition.
Other information
- •
- each capsule contains: sodium 303 mg
- •
- read the directions, warnings and accompanying label information before use
- •
- store at 20-25°C (68-77°F)
- •
- tamper-evident: Do not use if the band around the capsule is missing or broken. Do not use if printed seal under cap is broken or missing.
- •
- keep product out of high heat and humidity
- •
- protect product from moisture