Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- toothache
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
When using this product do not exceed recommended dose (see overdose warning)
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
| Weight (lb) | Age (yr) | Dose (tablets) * |
|---|---|---|
|
||
| under 24 | under 2 years | ask a doctor |
| 24-35 lbs | 2-3 years | 1 tablet |
| 36-47 lbs | 4-5 years | 1½ tablets |
| 48-59 lbs | 6-8 years | 2 tablets |
| 60-71 lbs | 9-10 years | 2½ tablets |
| 72-95 lbs | 11 years | 3 tablets |
Other information
- store between 20-25°C (68-77°F). Avoid high humidity. Protect from light.
- do not use if neck band or foil inner seal imprinted with "TYLENOL" is broken or missing
Inactive ingredients
anhydrous citric acid, cellulose acetate, crospovidone, D&C red no. 7 calcium lake, D&C red no. 30 aluminum lake, dextrose, FD&C blue no. 1 aluminum lake, flavor, magnesium stearate, povidone, sucralose
Uses
temporarily:
- reduces fever
- relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- toothache
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
When using this product do not exceed recommended dose (see overdose warning)
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- shake well before using
- mL = milliliter
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- remove the child protective cap and squeeze your child's dose into the dosing cup
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
| Weight (lb) | Age (yr) | Dose (mL) * |
|---|---|---|
|
||
| under 24 | under 2 years | ask a doctor |
| 24-35 lbs | 2-3 years | 5 mL |
| 36-47 lbs | 4-5 years | 7.5 mL |
| 48-59 lbs | 6-8 years | 10 mL |
| 60-71 lbs | 9-10 years | 12.5 mL |
| 72-95 lbs | 11 years | 15 mL |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
Other information
- each 5 mL contains: sodium 2 mg
- store between 20-25°C (68-77°F)
- do not use if carton is opened. Do not use if bottle wrap imprinted with “TYLENOL” is broken or missing
Inactive ingredients
anhydrous citric acid, D&C red no. 33, FD&C blue no. 1, flavors, glycerin, high fructose corn syrup, microcrystalline cellulose and carboxymethylcellulose sodium, purified water, sodium benzoate, sorbitol solution, sucralose, xanthan gum
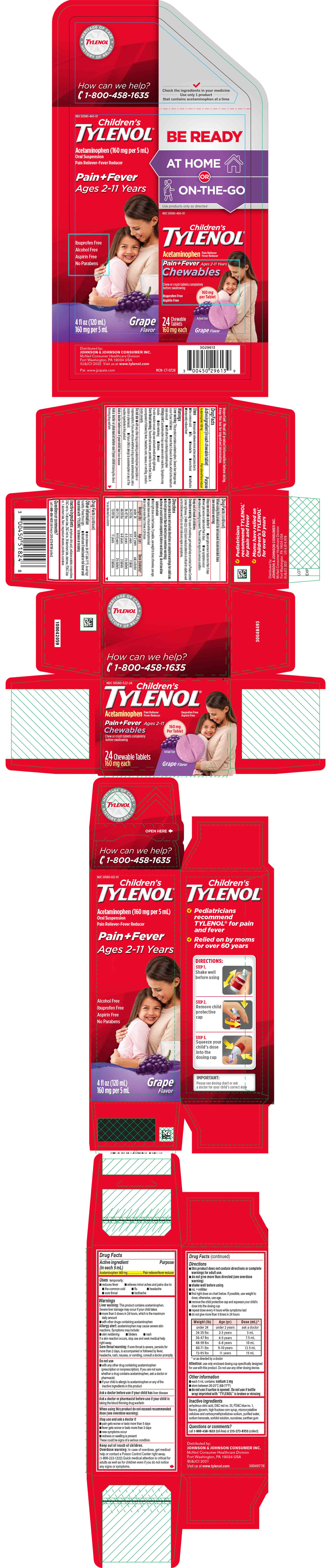
PRINCIPAL DISPLAY PANEL
NDC 50580-460-01
Children's
TYLENOL
®
Acetaminophen (160 mg per 5 mL)
Oral Suspension
Pain Reliever-Fever Reducer
Pain+Fever
Ages 2-11 Years
Ibuprofen Free
Alcohol Free
Aspirin Free
No Parabens
4 fl oz (120 mL)
160 mg per 5 mL
Grape
Flavor
BE READY
AT HOME
OR
ON-THE-GO
Use products only as directed
NDC 50580-460-01
Children's
TYLENOL
®
Acetaminophen
Pain Reliever
Fever Reducer
Pain+Fever
Ages 2-11
Chewables
Chew or crush tablets completely
before swallowing
Ibuprofen Free
Aspirin Free
160 mg
Per Tablet
24
Chewable
Tablets
Actual Size
160 mg each
Grape Flavor