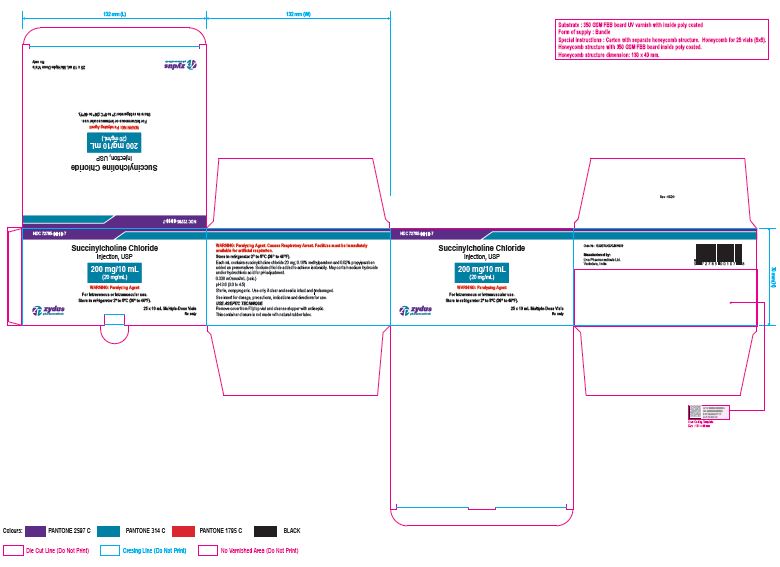
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 72785-0010-1
Succinylcholine Chloride Injection, USP
200 mg/10 mL (20 mg/mL)
For Intravenous or Intramuscular use.
10 mL Multiple-dose Vial
Rx only
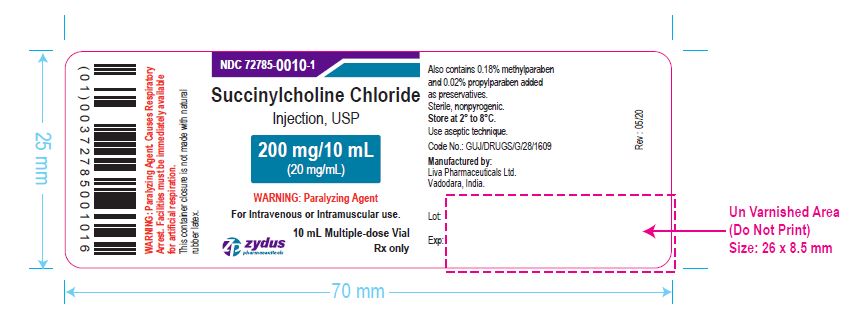
NDC 72785-0010-7
Succinylcholine Chloride Injection, USP
200 mg/10 mL (20 mg/mL)
For Intravenous or Intramuscular use.
Store in refrigerator 2º to 8ºC (36º to 46ºF).
25 x 10 mL Multiple-dose Vial
Rx only