FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Nitazoxanide Tablets are indicated for the treatment of diarrhea caused by Giardia lamblia or Cryptosporidium parvum in patients 12 years and older.
Limitations of Use
Nitazoxanide Tablets have not been shown to be effective for the treatment of diarrhea caused by Cryptosporidium parvum in HIV-infected or immunodeficient patients [ see Clinical Studies (14.2)].
2 DOSAGE AND ADMINISTRATION
The recommended dosage of Nitazoxanide Tablets in patient 12 years and older is 500 mg orally every 12 hours with food for 3 days.
Nitazoxanide Tablets should not be administered to pediatric patients 11 years of age or younger because a single tablet contains a greater amount of nitazoxanide than the recommended dosing in this pediatric age group. Nitazoxanide Tablets are not interchangeable with Nitazoxanide for Oral Suspension [see Clinical Pharmacology (12.3)]
3 DOSAGE FORMS AND STRENGTHS
Round, yellow, film-coated tablets debossed with 77 on one side and plain on the other side. Each tablet contains 500 mg of nitazoxanide.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of nitazoxanide was evaluated in 2177 HIV-uninfected subjects who received nitazoxanide at the recommended dose for at least three days. In pooled controlled clinical trials involving 536 HIV-uninfected subjects treated with nitazoxanide, the most common adverse reactions were abdominal pain, headache, chromaturia and nausea (≥2%).
Safety data were analyzed separately for 280 HIV-uninfected subjects ≥12 years of age receiving nitazoxanide at the recommended dose for at least three days in 5 placebo-controlled clinical trials.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of nitazoxanide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following is a list of adverse reactions spontaneously reported with Nitazoxanide Tablets which were not included in clinical trial listings:
Gastrointestinal disorders: diarrhea, gastroesophageal reflux disease
Nervous System disorders: dizziness
Respiratory, thoracic and mediastinal disorders: dyspnea
Skin and subcutaneous tissue disorders: rash, urticaria
7 DRUG INTERACTIONS
7.1 Highly Protein Bound Drugs with Narrow Therapeutic Indices
Tizoxanide (the active metabolite of nitazoxanide) is highly bound to plasma protein (>99.9%). Therefore, monitor for adverse reactions when administering nitazoxanide concurrently with other highly plasma protein-bound drugs with narrow therapeutic indices, as competition for binding sites may occur (e.g., warfarin).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with nitazoxanide in pregnant women to inform a drug-associated risk. No teratogenicity or fetotoxicity was observed in animal reproduction studies with administration of nitazoxanide to pregnant rats and rabbits during organogenesis at exposure 30 and 2 times, respectively, the exposure at the maximum recommended human dose of 500 mg twice daily based on body surface area (BSA).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Nitazoxanide was administered orally to pregnant rats at doses of 0, 200, 800 or 3200 mg/kg/day on gestation days 6 to 15. Nitazoxanide produced no evidence of systemic maternal toxicity when administer once daily via oral gavage to pregnant female rats at levels up to 3200 mg/kg/day during the period of organogenesis .
In rabbits, nitazoxanide was administered at doses of 0, 25, 50, or 100 mg/kg/day on gestation days 7 to 20. Oral treatment of pregnant rabbits with nitazoxanide during organogenesis resulted in minimal maternal toxicity and no external fetal anomalies.
8.2 Lactation
Risk Summary
No information regarding the presence of nitazoxanide in human milk, the effects on the breastfed infant, or the effects on milk production is available. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for nitazoxanide and any potential adverse effects on the breastfed infant from nitazoxanide or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of Nitazoxanide Tablets for the treatment of diarrhea caused by G. lamblia or C. parvum in pediatric patients 12 to 17 years of age has been established based on three (3) randomized, controlled studies with 47 pediatric subjects treated with Nitazoxanide Tablets [ see Clinical Studies (14)].
A single Nitazoxanide Tablet contains a greater amount of nitazoxanide than is recommended for use in pediatric patients 11 years or younger. Therefore, Nitazoxanide Tablets should not be administered to peridatric patients 11 years of age or younger [ see Dosage and Administration (2)].
8.5 Geriatric Use
Clinical studies of Nitazoxanide Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing Nitazoxanide Tablets.
8.6 Renal and Hepatic Impairment
The pharmacokinetics of nitazoxanide in patients with compromised renal or hepatic function has not been studied.
8.7 HIV-Infected or Immunodeficient Patients
Nitazoxanide Tablets have not been studied for the treatment of diarrhea caused by G. lamblia in HIV-infected or immunodeficient patients. Nitazoxanide Tablets have not been shown to be superior to placebo for the treatment of diarrhea caused by C. parvum in HIV-infected or immunodeficient patients [ see Clinical Studies (14)].
10 OVERDOSAGE
Limited information on nitazoxanide overdosage is available. In the event of overdose, gastric lavage may be appropriate soon after oral administration. Patients should be observed and given symptomatic and supportive treatment. There is no specific antidote for overdose with nitazoxanide. Because tizoxanide is highly protein bound (>99.9%), dialysis is unlikely to significantly reduce plasma concentrations of the drug.
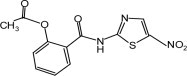
11 DESCRIPTION
Nitazoxanide Tablets contain the active ingredient, nitazoxanide, a synthetic antiprotozoal for oral administration. Nitazoxanide is a light yellow crystalline powder. It is poorly soluble in ethanol and practically insoluble in water. Chemically, nitazoxanide is 2-acetyloxy- N-(5-nitro-2-thiazolyl)benzamide. The molecular formula is C 12H 9N 3O 5S and the molecular weight is 307.3. The structural formula is:
Nitazoxanide Tablets contain 500 mg of nitazoxanide and the following inactive ingredients: maize starch, pregelatinized corn starch, hydroxypropyl methylcellulose, sucrose, sodium starch glycollate, talc, magnesium stearate, soy lecithin, polyvinyl alcohol, xanthan gum, titanium dioxide, D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, and FD&C Blue No. 2 Aluminum Lake.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
Single Dosing:
Following oral administration of Nitazoxanide Tablet, the parent drug, nitazoxanide, is not detected in plasma. The pharmacokinetic parameters of the metabolites, tizoxanide and tizoxanide glucuronide are shown in Table 1 below.
Table 1. Mean (+/- SD) plasma pharmacokinetic parameters of tizoxanide and tizoxanide glucuronide following administration of a single dose of one 500 mg Nitazoxanide Tablet with food to subjects ≥12 years of age
| Tizoxanide | Tizoxanide Glucuronide | |||||
| Age | C max (µg/mL) | T max (hr)* | AUC t (µg•hr/mL) | C max (µg/mL) | T max (hr)* | AUC t (µg•hr/mL) |
| 12 - 17 years | 9.1 (6.1) | 4.0 (1-4) | 39.5 (24.2) | 7.3 (1.9) | 4.0 (2-8) | 46.5 (18.2) |
| >18 years | 10.6 (2.0) | 3.0 (2-4) | 41.9 (6.0) | 10.5 (1.4) | 4.5 (4-6) | 63.0 (12.3) |
*T max is given as a Mean (Range)
Multiple dosing:
Following oral administration of a single Nitazoxanide Tablet every 12 hours for 7 consecutive days, there was no significant accumulation of nitazoxanide metabolites tizoxanide or tizoxanide glucuronide detected in plasma.
Bioavailability:
Nitazoxanide for oral suspension is not bioequivalent to Nitazoxanide Tablets. The relative bioavailability of the suspension compared to the tablet was 70%.
When Nitazoxanide Tablets are administered with food, the AUC t of tizoxanide and tizoxanide glucuronide in plasma is increased almost two-fold and the C max is increased by almost 50%.
Nitazoxanide Tablets were administered with food in clinical trials and hence they are recomended to be administered with food [see Dosage and Administration (2.1)]
Distribution
In plasma, more than 99% of tizoxanide is bound to proteins.
Elimination
Metabolism
Following oral administration in humans, nitazoxanide is rapidly hydrolyzed to an active metabolite, tizoxanide (desacetyl-nitazoxanide). Tizoxanide then undergoes conjugation, primarily by glucuronidation.
Excretion
Tizoxanide is excreted in the urine, bile and feces, and tizoxanide glucuronide is excreted in urine and bile. Approximately two-thirds of the oral dose of nitazoxanide is excreted in the feces and one-third in the urine.
Specific Populations
Pediatric Patients
The pharmacokinetics of tizoxanide and tizoxanide glucuronide following administration of Nitazoxanide Tablets in pediatric patients 12-17 years of age are provided above in Table 1.
Drug Interaction Studies
In vitro studies have demonstrated that tizoxanide has no significant inhibitory effect on cytochrome P450 enzymes.
12.4 MICROBIOLOGY
Mechanism of Action
The antiprotozoal activity of nitazoxanide is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. Studies have shown that the PFOR enzyme from G. lamblia directly reduces nitazoxanide by transfer of electrons in the absence of ferredoxin. The DNA-derived PFOR protein sequence of C. parvum appears to be similar to that of G. lamblia. Interference with the PFOR enzyme-dependent electron transfer reaction may not be the only pathway by which nitazoxanide exhibits antiprotozoal activity.
Resistance
A potential for development of resistance by C. parvum or G. lamblia to nitazoxanide has not been examined.
Antimicrobial Activity
Nitazoxanide and its metabolite, tizoxanide, are active in vitro in inhibiting the growth of (i) sporozoites and oocysts of C. parvum and (ii) trophozoites of G. lamblia.
Susceptibility Test Methods
For protozoa such as C. parvum and G. lamblia, standardized tests for use in clinical microbiology laboratories are not available.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies have not been conducted.
Mutagenesis
Nitazoxanide was not genotoxic in the Chinese hamster ovary (CHO) cell chromosomal aberration assay or the mouse micronucleus assay. Nitazoxanide was genotoxic in one tester strain (TA 100) in the Ames bacterial mutation assay.
Impairment of Fertility
Nitazoxanide did not adversely affect male or female fertility in the rat at 2400 mg/kg/day (approximately 20 times the clinical adult dose adjusted for body surface area).
14 CLINICAL STUDIES
14.1 Diarrhea Caused by G. lamblia
Diarrhea caused by G. lamblia in adults and adolescents 12 years of age or older:
In a double-blind, controlled trial (Study 1) conducted in Peru and Egypt in adults and adolescents with diarrhea and with one or more enteric symptoms (e.g., abdominal pain, nausea, vomiting, fever, abdominal distention, loss of appetite, flatulence) caused by G. lamblia, a three-day course of treatment with Nitazoxanide Tablets administered 500 mg BID was compared with a placebo tablet for 3 days. A second double-blind, controlled trial (Study 2) conducted in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., abdominal colic, abdominal tenderness, abdominal cramps, abdominal distention, fever, bloody stool) caused by G. lamblia compared Nitazoxanide Tablets administered 500 mg BID for 3 days to a placebo tablet. For both of these studies, clinical response was evaluated 4 to 7 days following the end of treatment. A clinical response of ‘well’ was defined as ‘no symptoms, no watery stools and no more than 2 soft stools with no hematochezia within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 2. Adult and Adolescent Patients with Diarrhea Caused by G. lamblia
Clinical Response Rates* 4 to 7 Days Post-therapy
% (Number of Successes/Total)
|
Nitazoxanide Tablets |
Placebo Tablets |
||
|
Study 1 |
85% (46/54) ¶ |
44% (12/27) |
|
|
Study 2 |
100% (8/8) |
30% (3/10) |
* Includes all patients randomized with G. lamblia as the sole pathogen. Patients failing to complete the studies were treated as failures.
¶ Clinical response rates statistically significantly higher when compared to placebo.
Some patients with ‘well’ clinical responses had G. lamblia cysts in their stool samples 4 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
14.2 Diarrhea Caused by C. parvum
Diarrhea caused by C. parvum in adults and adolescents 12 years of age or older:
In a double-blind, controlled trial conducted in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., abdominal pain/cramps, nausea, vomiting) caused by C. parvum, a three-day course of treatment with Nitazoxanide Tablets administered 500 mg BID was compared with a placebo tablet for 3 days. Clinical response was evaluated 4 to 7 days following the end of treatment. A clinical response of ‘well’ was defined as ‘no symptoms, no watery stools and no more than 2 soft stools within the past 24 hours’ or ‘no symptoms and no unformed stools within the past 48 hours.’ The following clinical response rates were obtained:
Table 3. Clinical Response Rates in Adult and Adolescent Patients 4 to 7 Days Post-therapy
% (Number of Successes/Total)
|
Nitazoxanide Tablets |
Placebo Tablets |
||
|
Intent-to-treat analysis* |
96% (27/28) ¶ |
41% (11/27) |
* Includes all patients randomized with C. parvum as the sole pathogen. Patients failing to complete the study were treated as failures.
¶ Clinical response rates statistically significantly higher when compared to placebo.
In a second double-blind, placebo-controlled trial of Nitazoxanide Tablets conductd in Egypt in adults and adolescents with diarrhea and with or without enteric symptoms (e.g., addominal colic, abdominal cramps, epigastric pain) caused by C. parvum as the sole pathogen, clinical and parasitological reponse rates showed a similar trend to the first study. Clinical response rates, evaluted 2 to 6 days following the end of treatment, were 71% (15/21) in the nitazoxanide group and 42.9% (9/21) in the placebo group.
Some patients with ‘well’ clinical responses had C. parvum oocysts in their stool samples 4 to 7 days following the end of treatment. The relevance of stool examination results in these patients is unknown. Patients should be managed based upon clinical response to treatment.
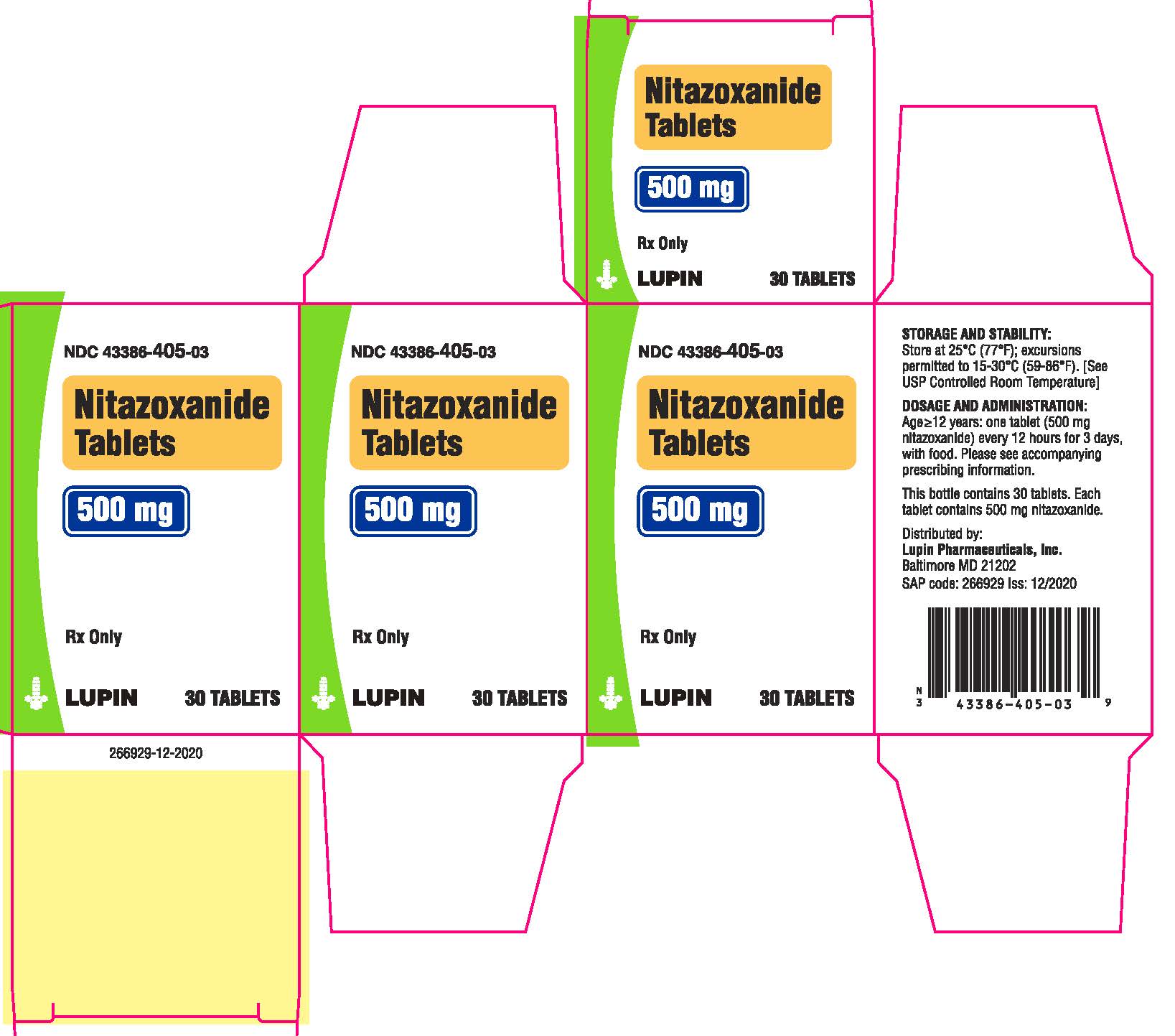
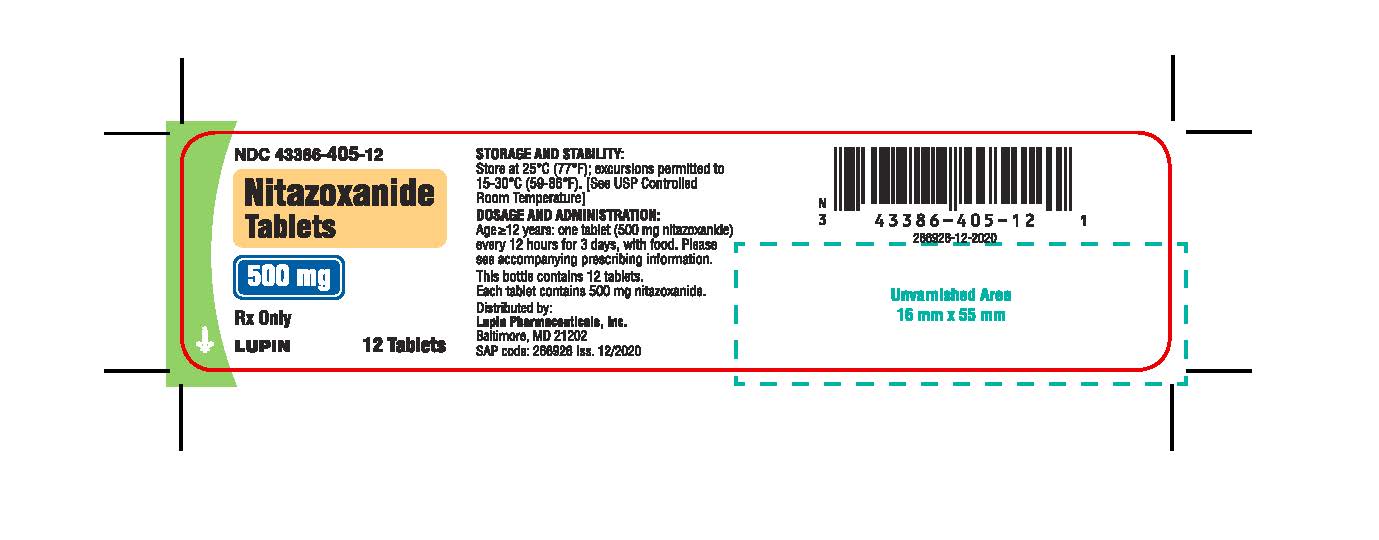
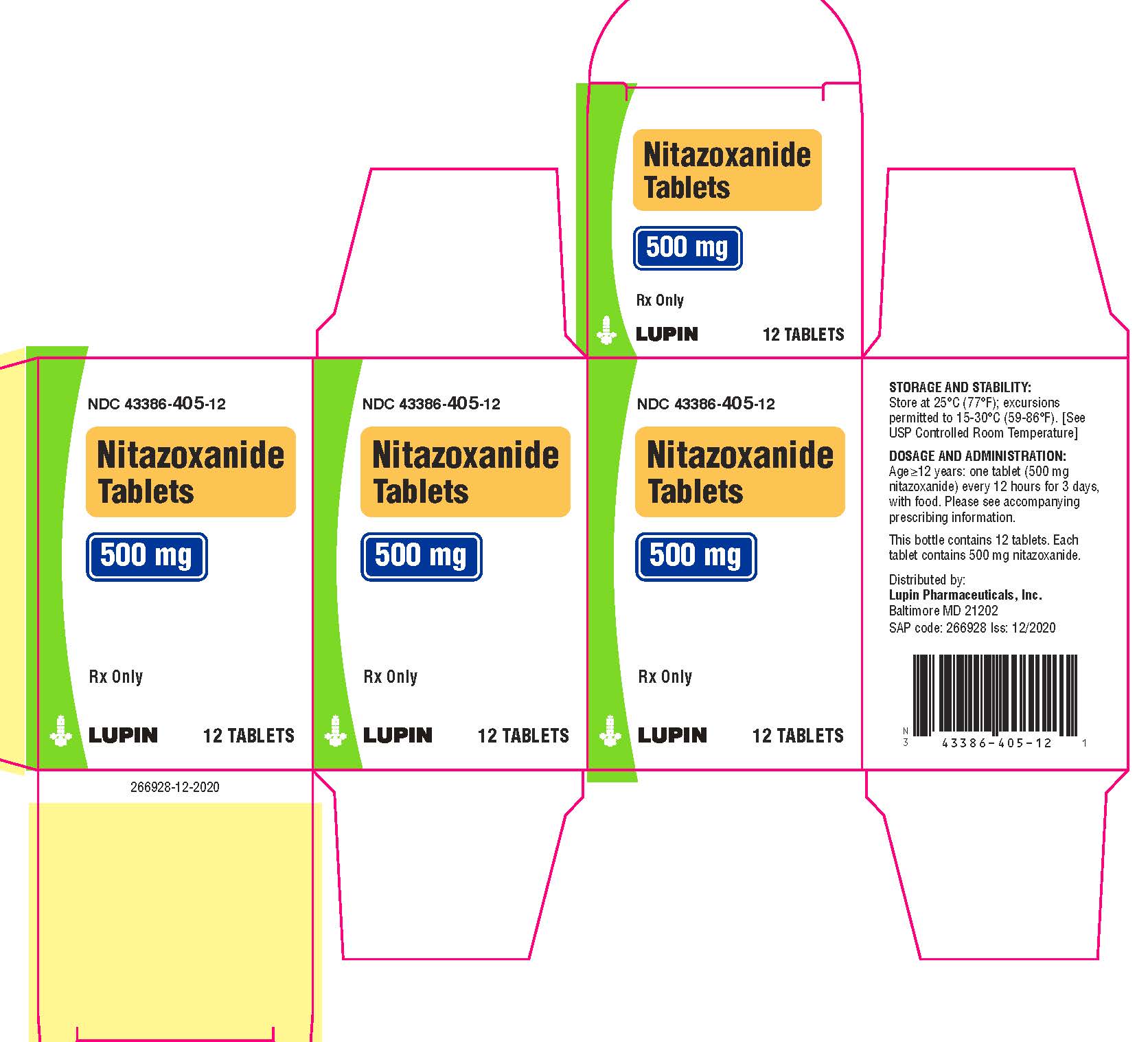
16 HOW SUPPLIED/STORAGE AND HANDLING
Nitazoxanide Tablets are round, yellow, film-coated tablets debossed with 77 on one side and plain on the other side. Each tablet contains 500 mg of nitazoxanide. The tablets are packaged in HDPE bottles of 12 and 30 tablets.
Bottles of 12 tablets NDC 43386-405-12
Bottles of 30 tablets NDC 43386-405-03
Store the tablets at 25 oC (77 oF); excursions permitted to 15 oC-30 oC (59 oF-86 oF). [See USP Controlled Room Temperature]
17 PATIENT COUNSELING INFORMATION
Advise patients and parents/caregivers of pediatric patients taking Nitazoxanide Tablets of the following information:
Dosage and Administration:
Nitazoxanide Tablets should be taken with food.
Drug-drug Interactions:
Avoid concurrent warfarin use.
MANUFACTURER INFORMATION
Manufactured By:
Romark Laboratorties, L.C.
Tampa, FL 33607
Telephone: 813-282-8544, Fax: 813-282-1162
E-mail:
customer.service@romark.com
Website: www.romark.com
Distributed by:
Lupin Pharmaceuticals, Inc.
Baltimore, MD 21202
SAP code: 266925 Iss.: 12/2021
Nitazoxanide Tablets - 30 ct Label
NDC 43386-405-03
Nitazoxanide Tablets
500 mg
Rx Only
LUPIN 30 TABLETS
Nitazoxanide Tablets - 30 ct Carton
NDC 43386-405-03
Nitazoxanide Tablets
500 mg
Rx Only
LUPIN 30 Tablets