FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Mannitol Injection is indicated for the reduction of:
- intracranial pressure and treatment of cerebral edema;
- elevated intraocular pressure.
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Mannitol Injection is for intravenous infusion preferably into a large central vein [see Warnings and Precautions (5.6), Description (11)].
- Prior to administration of Mannitol Injection, evaluate renal, cardiac, and pulmonary status of the patient and correct fluid and electrolyte imbalances [see Dosage and Administration (2.2)].
- Do not administer Mannitol Injection simultaneously with blood products through the same administration set because of the possibility of pseudoagglutination or hemolysis.
Preparation
- Tear outer wrap at notch and remove solution container.
- Visually inspect flexible container. Do not administer unless the solution is clear and the seal is intact.
- If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired.
- Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
- Check for minute leaks prior to use by squeezing the bag firmly. If leaks are detected, discard solution as sterility may be impaired.
- Admixing Mannitol Injection with other medications is not recommended.
- Inspect Mannitol Injection visually for particulate matter and discoloration prior to administration. If particulates or discoloration are present, discard the bag.
Manitol Injection solutions may crystalize when exposed to low temperatures. Inspect Mannitol Injection for crystals prior to administration. If crystals are visible, re-dissolve by warming the solution up to 70°C with agitation. Heat solution by using a dry-heat cabinet with overwrap intact. The use of a water bath is not recommended. Allow the solution to cool to body temperature or less before administering. Reinspect Mannitol Injection for crystals prior to administration. Discard the solution if all the crystals cannot be dissolved.
Administration
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
- Use administration sets with a final in-line filter because of the potential for mannitol crystals to form.
- Suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Open flow control clamp and clear air from set. Close clamp.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- Regulate rate of administration with flow control clamp.
- To prevent air embolism, use a non-vented infusion set or close the vent on a vented set, avoid multiple connections, do not connect flexible containers in series, fully evacuate residual gas in the container prior to administration, do not pressurize the flexible container to increase flow rates, and if administration is controlled by pumping device, turn off pump before the container runs dry.
- For single use only; discard unused portion.
2.2 Recommended Dosage
Prior to administration of Mannitol Injection, evaluate renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances.
The total dosage, concentration, and rate of administration depend on the age, weight, and condition of the patient being treated, including fluid requirement, electrolyte balance, serum osmolality, urinary output, and concomitant therapy.
The following outline of administration and dosage is only a general guide to therapy.
Reduction of Intracranial Pressure and Treatment of Cerebral Edema
Usually a maximum reduction in intracranial pressure can be achieved with a dose of 0.25 gram/kg given intravenously as an intravenous infusion over at least 30 minutes, which may be repeated every six to eight hours.
During and following Mannitol Injection infusion, monitor fluid and electrolytes, serum osmolarity, and renal, cardiac and pulmonary function. Discontinue Mannitol Injection if renal, cardiac, or pulmonary status worsens or CNS toxicity develops [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
Reduction of Intraocular Pressure
The recommended dosage is 1.5 to 2 g/kg as a single dose administered as an intravenous infusion over at least 30 minutes. When used preoperatively, administer Mannitol Injection 60 to 90 minutes before surgery to achieve maximal reduction of intraocular pressure before operation.
3 DOSAGE FORMS AND STRENGTHS
Injection: 20% (0.2 grams/mL); 20 grams of mannitol, USP per 100 mL as a clear and colorless solution in single-dose 250 mL and 500 mL flexible containers
4 CONTRAINDICATIONS
Mannitol Injection is contraindicated in patients with:
- Known hypersensitivity to mannitol [see Warnings and Precautions (5.1)]
- Anuria [see Warnings and Precautions (5.2)]
- Severe hypovolemia [see Warnings and Precautions (5.4)]
- Pre-existing pulmonary vascular congestion or pulmonary edema [see Warnings and Precautions (5.5)]
- Active intracranial bleeding except during craniotomy.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, hypotension and dyspnea resulting in cardiac arrest and death have been reported with Mannitol Injection [see Adverse Reactions (6)].
Stop the infusion immediately if signs or symptoms of a suspected hypersensitivity reaction develop. Initiate appropriate therapeutic countermeasures as clinically indicated.
5.2 Renal Complications Including Renal Failure
Renal complications, including irreversible renal failure have been reported in patients receiving mannitol. Reversible, oliguric acute kidney injury has occurred in patients with normal pretreatment renal function who received large intravenous doses of mannitol. Although the osmotic nephrosis associated with mannitol administration is in principle reversible, osmotic nephrosis in general is known to potentially proceed chronic or even end-stage renal failure. Monitor renal function closely, including signs of urine output reduction, during Mannitol Injection infusion. Patients with pre-existing renal disease, patients with conditions that put them at high risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure following administration of Mannitol Injection. Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) or other diuretics with Mannitol Injection, if possible [see Drug Interactions (7.1, 7.2)].
Patients with oliguric acute kidney injury who subsequently develop anuria while receiving mannitol are at risk of congestive heart failure, pulmonary edema, hypertensive crisis, coma and death.
During and following Mannitol Injection infusion for the reduction in intracranial pressure, monitor the patient clinically and review laboratory tests for changes in fluid and electrolyte status. Discontinue Mannitol Injection if renal function worsens [see Warnings and Precautions (5.5)].
5.3 Central Nervous System (CNS) Toxicity
CNS toxicity manifested by, e.g., confusion, lethargy, coma has been reported in patients treated with mannitol, some resulting in death, in particular in the presence of impaired renal function CNS toxicity may result from high serum mannitol concentrations, serum hyperosmolarity resulting in intracellular dehydration within CNS, hyponatremia or other disturbances of electrolyte and acid/base balance secondary to mannitol administration [see Warnings and Precautions (5.4)].
At high concentrations, mannitol may cross the blood brain barrier and interfere with the ability of the brain to maintain the pH of the cerebrospinal fluid especially in the presence of acidosis.
In patients with preexisting compromise of the blood brain barrier, the risk of increasing cerebral edema (general and focal) associated with repeated or continued use of Mannitol Injection must be individually weighed against the expected benefits.
A rebound increase of intracranial pressure may occur several hours after the infusion. Patients with a compromised blood brain barrier are at increased risk.
Concomitant administration of neurotoxic drugs (e.g., aminoglycosides) with Mannitol Injection may potentiate neurotoxicity. Avoid concomitant use of neurotoxic drugs, if possible [see Drug Interactions (7.3)].
During and following Mannitol Injection infusion for the reduction in intracranial pressure, monitor the patient clinically and laboratory tests for changes in fluid and electrolyte status. Discontinue Mannitol Injection if CNS toxicity develops [see Warnings and Precautions (5.5)].
5.4 Fluid and Electrolyte Imbalances, Hyperosmolarity
Depending on dosage and duration, administration of Mannitol Injection may result in hypervolemia leading to or exacerbating existing congestive heart failure. Accumulation of mannitol due to insufficient renal excretion increases the risk of hypervolemia.
Mannitol-induced osmotic diuresis may cause or worsen dehydration/hypovolemia and hemoconcentration. Administration of Mannitol Injection may also cause hyperosmolarity [see Description (11)].
Depending on dosage and duration of administration, electrolyte and acid/base imbalances may also result from transcellular shifts in water and electrolytes, osmotic diuresis and/or other mechanisms. Such imbalances may be severe and potentially fatal.
Imbalances that may result from Mannitol Injection administration include:
- Hypernatremia, dehydration and hemoconcentration
- Hyponatremia, which can lead to headache, nausea, seizures, lethargy, coma, cerebral edema, and death. Acute symptomatic hyponatremic encephalopathy is considered a medical emergency.
- Hypo/hyperkalemia. The development of electrolyte imbalances (e.g., hyperkalemia, hypokalemia) associated with mannitol administration may result in cardiac adverse reactions in patients receiving drugs that are sensitive to such imbalances (e.g., digoxin, agents that may cause QT prolongation, neuromuscular blocking agents) [see Drug Interactions (7.4)].
- Other electrolyte disturbances
- Metabolic acidosis/alkalosis
Pediatric patients less than two years of age, particularly preterm and term neonates, may be at higher risk for fluid and electrolyte abnormalities following Mannitol Injection administration due to decreased glomerular filtration rate and limited ability to concentrate urine [see Use in Specific Populations (8.4)].
During and following Mannitol Injection infusion for the reduction in intracranial pressure, monitor fluid, acid-base balance and electrolyte status and discontinue Mannitol Injection if imbalances occur [see Warnings and Precautions (5.5)].
5.5 Monitoring/Laboratory Tests
During and following infusion of Mannitol Injection for reduction of intracranial pressure and monitor:
- serum osmolarity, serum electrolytes (sodium, potassium, calcium, and phosphate) and acid base balance
- the osmol gap
- signs and symptoms of hypo- or hypervolemia, including urine output
- renal, cardiac, and pulmonary function
- intracranial pressure
Discontinue Mannitol Injection if renal, cardiac, or pulmonary status worsens or CNS toxicity develops [see Contraindications (4)].
5.6 Infusion Site Reactions
The infusion of hypertonic solutions through a peripheral vein, including Mannitol Injection may result in peripheral venous irritation, including phlebitis. Other severe infusion site reactions, such as compartment syndrome and swelling associated with extravasation, can occur with administration of Mannitol Injection [see Adverse Reactions (6)]. Administration through a large central vein is preferred with mannitol [see Dosage and Administration (2.1)].
5.7 Interference with Laboratory Tests
High concentrations of mannitol can cause false low results for inorganic phosphorus blood concentrations [see Drug Interactions (7.6)].
Mannitol may produce false positive results in tests for blood ethylene glycol concentrations [see Drug Interactions (7.6)].
6 ADVERSE REACTIONS
The following adverse reactions from voluntary reports or clinical studies have been reported with Mannitol Injection. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions:, anaphylaxis, hypotension, dyspnea, hypertension, pyrexia, chills, sweating, urticaria/rash, pruritus [see Warnings and Precautions (5.1)]
- Renal Failure: acute kidney injury, osmotic nephrosis, azotemia, anuria, hematuria, oliguria, polyuria, urinary retention [see Warnings and Precautions (5.2)]
- CNS Toxicity: coma, seizures, confusion, lethargy; rebound increase in intracranial pressure; dizziness, headache [see Warnings and Precautions (5.3)]
- Fluid and Electrolyte Imbalances: hypovolemia (dehydration), hypervolemia, peripheral edema, , hyponatremia, hypernatremia, hyperkalemia, hypokalemia; metabolic acidosis [see Warning and Precautions (5.4)]
- Infusion Site Reactions: venous thrombosis, phlebitis, inflammation, pain, rash, erythema, pruritus; compartment syndrome and swelling associated with extravasation [see Warnings and Precautions (5.6)]
- Cardiac and Respiratory Disorders: cardiac arrest, congestive cardiac failure, pulmonary edema, palpitations, tachycardia, angina-like chest pain
- Musculoskeletal Disorders: musculoskeletal stiffness, myalgia
- Gastrointestinal Disorders: thirst, dry mouth, nausea, vomiting
- General Disorders: asthenia, malaise
7 DRUG INTERACTIONS
7.1 Nephrotoxic Drugs
Concomitant administration of nephrotoxic drugs (e.g., aminoglycosides, cyclosporine) increases the risk of renal failure following administration of mannitol. Avoid use of nephrotoxic drugs with Mannitol Injection, if possible [see Warnings and Precautions (5.2)].
7.2 Diuretics
Concomitant administration of other diuretics may potentiate the renal toxicity of mannitol. Avoid use of other diuretics with Mannitol Injection, if possible [see Warnings and Precautions (5.2)].
7.3 Neurotoxic Drugs
Concomitant administration of systemic neurotoxic drugs (e.g., aminoglycosides) with Mannitol Injection may potentiate the CNS toxicity of mannitol. Avoid concomitant administration of neurotoxic drugs with mannitol [see Warnings and Precautions (5.3)].
7.4 Drugs Affected by Electrolyte Imbalances
The development of electrolyte imbalances (e.g., hyperkalemia, hypokalemia) associated with mannitol administration may result in cardiac adverse reactions in patients receiving drugs that are sensitive to such imbalances (e.g., digoxin, drugs that prolong the QT interval, neuromuscular blocking agents) [see Warnings and Precautions (5.4)]. During and following Mannitol Injection infusion, monitor serum electrolytes and discontinue Mannitol Injection if cardiac status worsens [see Warnings and Precautions (5.5)].
7.5 Renally Eliminated Drugs
Mannitol may increase the elimination, and decrease the effectiveness of treatment with, drugs that undergo significant renal elimination. Concomitant administration of mannitol with lithium may initially increase the elimination of lithium but may also increase the risk of lithium toxicity in patients who develop hypovolemia or renal impairment. Consider holding lithium therapy during treatment with Mannitol Injection. If lithium therapy cannot be held, monitor serum lithium concentrations frequently for signs of lithium toxicity. Mannitol therapy may increase the elimination of digoxin leading to a potential decrease in effectiveness of the treatment. Monitor digoxin serum concentrations.
7.6 Interference with Laboratory Tests
High concentrations of mannitol can cause false low results for inorganic phosphorus blood concentrations when an assay based on the conversion of phosphate (orthophosphate) to the phosphomolybdate complex is used [see Warnings and Precautions (5.7)].
Mannitol may produce false positive results in tests for blood ethylene glycol concentrations in which mannitol is initially oxidized to an aldehyde [see Warnings and Precautions (5.7)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available case report data with mannitol over decades of use have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Mannitol crosses the placenta and may cause fluid shifts that could potentially result in adverse effects in the fetus (see Data). No adverse developmental effects from mannitol were reported in published animal studies; however, fluid shifts occurred in fetal ewes in response to maternal infusion of mannitol.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mannitol in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Mannitol Injection and any potential adverse effects on the breastfed infant from Injection or from the underlying maternal condition.
8.4 Pediatric Use
Mannitol Injection is approved for use in the pediatric population for the reduction of intracranial and intraocular pressure. Studies have not defined the optimal dose of Mannitol Injection in the pediatric population. The safety profile for mannitol use in pediatric patients is similar to adults at dosages described in labeling. However, pediatric patients less than two years of age, particularly preterm and term neonates, may be at higher risk for fluid and electrolyte abnormalities following Mannitol Injection administration due to decreased glomerular filtration rate and limited ability to concentrate urine [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
Mannitol is known to be substantially excreted by the kidney and the risk of adverse reactions to this drug may be greater in elderly patients with impaired renal function. Evaluate the renal, cardiac, and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of Mannitol Injection [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
8.6 Renal Impairment
Patients with pre-existing renal disease, patients with conditions that put them at high risk of renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure with administration of mannitol. Evaluate the renal, cardiac, and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of Mannitol Injection [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
10 OVERDOSAGE
Signs and symptoms of overdose with Mannitol Injection include renal failure and acute kidney injury, hypo/hypervolemia, hyperosmolarity and electrolyte imbalances, CNS toxicity (e.g., coma, seizures), some of which can be fatal [see Warnings and Precautions (5.2, 5.3, 5.4)].
Management of overdosage with Mannitol Injection is symptomatic and supportive. Discontinue the infusion and institute appropriate corrective measures with particular attention to renal, cardiac and pulmonary systems. Correct fluid and electrolyte imbalances.
Mannitol Injection is dialyzable (hemodialysis and peritoneal dialysis), hemodialysis may increase Mannitol Injection elimination.
11 DESCRIPTION
Mannitol Injection, USP is a sterile, nonpyrogenic solution of mannitol in water for injection available in a concentration of 20% in flexible plastic containers.
The content and characteristics of the available concentration is as follows:
| Concentration (%) | g/100 mL | mOsmol/liter (calc.) | pH* |
|---|---|---|---|
|
|||
| 20 | 20 | 1098 | 6.3 (4.5 to 7.0) |
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Mannitol Injection, USP is an osmotic diuretic for intravenous administration.
Mannitol, USP is chemically designated D-mannitol (C6H14O6), a white crystalline powder or free-flowing granules freely soluble in water. It has the following structural formula:
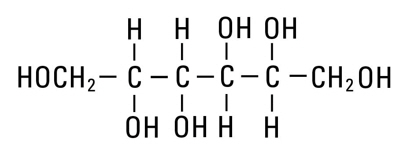
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap, but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mannitol, when administered intravenously, exerts its osmotic diuretic effect as a solute of relatively small molecular size being largely confined to the extracellular space. Mannitol hinders tubular reabsorption of water and enhances excretion of sodium and chloride by elevating the osmolarity of the glomerular filtrate.
This increase in extracellular osmolarity affected by the intravenous administration of mannitol will induce the movement of intracellular water to the extracellular and vascular spaces. This action underlies the role of mannitol in reducing intracranial pressure, intracranial edema, and intraocular pressure.
12.3 Pharmacokinetics
Distribution
Mannitol distributes largely in the extracellular space in 20 to 40 minutes after intravenous administration. The volume of distribution of mannitol is approximately 17 L in adults.
Elimination
In subjects with normal renal function, the total clearance is 87 to 109 mL/min. The elimination half-life of mannitol is 0.5 to 2.5 hours.
Metabolism
Only relatively small amount of the dose administered is metabolized after intravenous administration of mannitol to healthy subjects.
Excretion
Mannitol is eliminated primarily via the kidneys in unchanged form. Mannitol is filtered by the glomeruli, exhibits less than 10% of tubular reabsorption, and is not secreted by tubular cells. Following intravenous administration, approximately 80% of an administered dose of mannitol is estimated to be excreted in the urine in three hours with lesser amounts thereafter.
Specific Populations
Patients with Renal Impairment
In patients with renal impairment, the elimination half-life of mannitol is prolonged. In a published study, in patients with renal impairment including acute renal failure and end stage renal failure, the elimination half-life of mannitol was estimated at about 36 hours, based on serum osmolarity. In patients with renal impairment on dialysis, the elimination half-life of mannitol was reduced to 6 and 21 hours during hemodialysis and peritoneal dialysis, respectively. [see Use in Specific Populations (8.6), Overdosage (10)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Mannitol Injection is a clear and colorless solution supplied in single-dose containers as follows:
| Unit of Sale | Concentration | Each |
|---|---|---|
| ICU Medical is transitioning NDC codes from "0409" to "0990" labeler code. Both NDC codes are expected to be in the market for a period of time. | ||
| NDC 0409-7715-03 NDC 0990-7715-03 Case containing 12 | 20% (0.2 g/mL) mannitol, USP | NDC 0409-7715-13 NDC 0990-7715-13 500 mL Flexible Container |
| NDC 0409-7715-02 NDC 0990-7715-02 Case containing 24 | 20% (0.2 g/mL) mannitol, USP | NDC 0409-7715-12 NDC 0990-7715-12 250 mL Flexible Container |
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers, or home healthcare providers of the following risks of Mannitol Injection:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Renal Failure [see Warnings and Precautions (5.2)]
- CNS Toxicity [see Warnings and Precautions (5.3)]
- Fluid and Electrolyte Imbalances, Hyperosmolarity [see Warnings and Precautions (5.4)]
- Infusion Site Reactions [see Warnings and Precautions (5.6)]
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label
250 mL
NDC 0990-7715-12
20% Mannitol Injection,
USP
50 g/250 mL (200 mg/mL)
EACH 100 ML CONTAINS MANNITOL
20 GRAMS. MAY CONTAIN SODIUM
BICARBONATE FOR pH ADJUSTMENT.
1098 mOsmol/LITER (CALC.)
pH 6.3 (4.5 TO 7.0)
STERILE, NONPYROGENIC
ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF
AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR
INTRAVENOUS USE. USUAL DOSAGE:
SEE INSERT. WARNING: SEE INSERT
REGARDING CONTRAINDICATION IN
SEVERE RENAL IMPAIRMENT. WARNING:
IF CRYSTALS FORM, WARM WITH
OVERWRAP INTACT AND AGITATE TO
DISSOLVE. ADMINISTER INTRAVENOUSLY
AT 30° TO 37°C (86° TO 98.6°F). INSPECT
CAREFULLY. DISCARD UNUSED PORTION.
USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT
BE USED IN SERIES CONNECTIONS.
3
V
CONTAINS DEHP
Rx ONLY
IMP0000059
ICU Medical, Inc.,
Lake Forest, Illinois, 60045, USA
icumedical

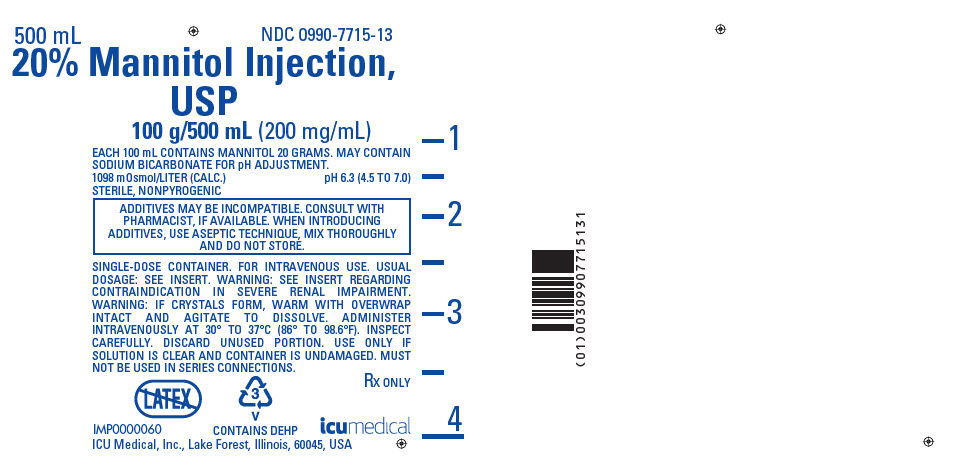
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label
500 mL
NDC 0990-7715-13
20% Mannitol Injection,
USP
100 g/500 mL (200 mg/mL)
EACH 100 mL CONTAINS MANNITOL 20 GRAMS. MAY CONTAIN
SODIUM BICARBONATE FOR pH ADJUSTMENT.
1098 mOsmol/LITER (CALC.)
pH 6.3 (4.5 TO 7.0)
STERILE, NONPYROGENIC
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH
PHARMACIST, IF AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE, MIX THOROUGHLY
AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR INTRAVENOUS USE. USUAL
DOSAGE: SEE INSERT. WARNING: SEE INSERT REGARDING
CONTRAINDICATION IN SEVERE RENAL IMPAIRMENT.
WARNING: IF CRYSTALS FORM, WARM WITH OVERWRAP
INTACT AND AGITATE TO DISSOLVE. ADMINISTER
INTRAVENOUSLY AT 30° TO 37°C (86° TO 98.6°F). INSPECT
CAREFULLY. DISCARD UNUSED PORTION. USE ONLY IF
SOLUTION IS CLEAR AND CONTAINER IS UNDAMAGED. MUST
NOT BE USED IN SERIES CONNECTIONS.
3
V
CONTAINS DEHP
Rx ONLY
IMP0000060
ICU Medical, Inc., Lake Forest, Illinois, 60045, USA
icumedical