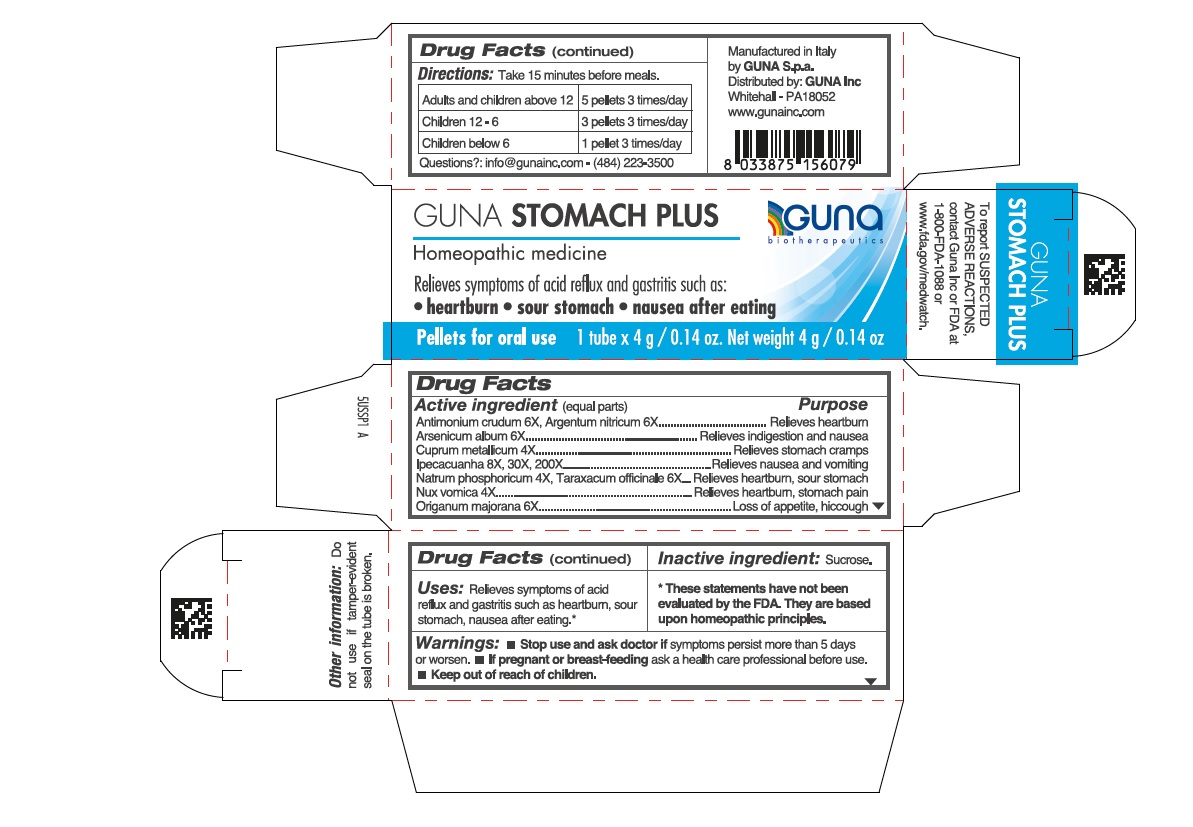
ACTIVE INGREDIENTS/PURPOSE
Antimonium crudum 6X Relieves heartburn
Argentum nitricum 6X Relieves heartburn
Arsenicum album 6X Relieves indigestion and nausea
Ipecacuanha 8X, 30X, 200X Relieves nausea and vomiting
Nux vomica 4X Relieves heartburn, stomach pain
Origanum majorana 6X Loss of appetite, hiccough
Cuprum metallicum 4X Relieves stomach cramps
Taraxacum officinale 6X Relieves heartburn, sour stomach
Natrum phosphoricum 4X Relieves heartburn, sour stomach
- Stop use and ask doctor if symptoms persist more than 5 days or worsen.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children.
USES
Relieves symptoms of acid reflux and gastritis such as:
- heartburn
- sour stomach
- nausea after eating
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days or worsen.
- If pregnant or breast-feeding ask a health care professional before use
- keep out of reach of children
DIRECTIONS
- Turn tube upside down and rotate cap to release pellets into cap. ● Unscrew cap and, without touching the pellets, place them under the tongue. ● Allow to dissolve. ● Take 15 minutes before meals.