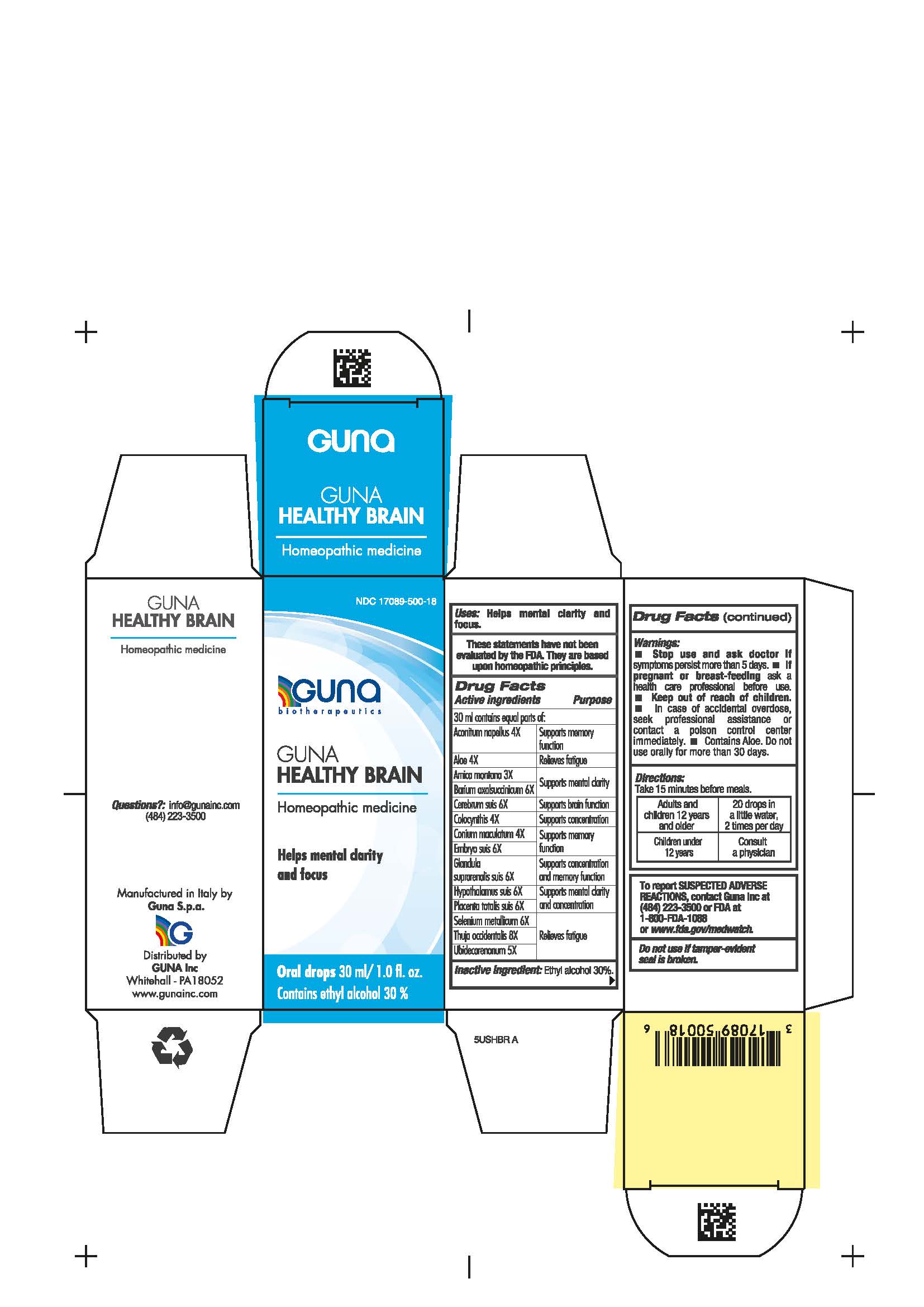
WARNINGS
- Stop use and ask doctor if symptoms worsen or persist more than 5 days.
- If pregnant or breast-feeding ask a health care professional before use.
- Keep out of reach of children.
- In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
- Contains Aloe. Do not use orally for more than 30 days.
- Contains ethyl alcohol 30%
DIRECTIONS
Take 15 minutes before meals.
Adults and children twelve years and older: 20 drops in a little water, 2 times per day
Children under 12 years: consult a physician
ACTIVE INGREDIENTS/PURPOSE
Aconitum napellus 4X Supports memory function
Aloe 4X Relieves fatigue
Arnica montana 3X Supports mental clarity
Barium oxalsuccinicum 6X Supports mental clarity
Cerebrum suis 6X Supports brain function
Colocynthis 4X Supports concentration
Conium maculatum 4X Supports memory function
Embryo suis 6X Supports memory function
Glandula suprarenalis suis 6X Supports concentration and memory function
Hypothalamus suis 6X Supports mental clarity and concentration
Placenta totalis suis 6X Supports mental clarity and concentration
Selenium metallicum 6X Relieves fatigue
Thuja occidentalis 8X Relieves fatigue
Ubidecarenonum 5X Relieves fatigue