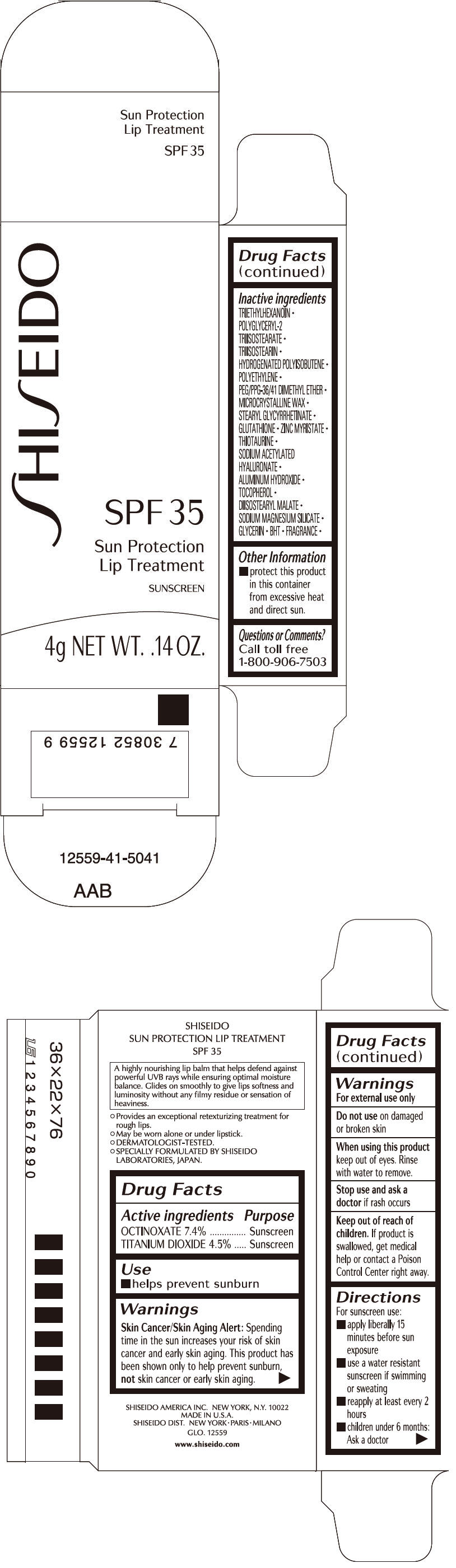
SHISEIDO SUN PROTECTION LIP TREATMENT- octinoxate and titanium dioxide lipstick
SHISEIDO AMERICA INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| ACTIVE INGREDIENTS: | Purpose |
| OCTINOXATE 7.4% | Sunscreen |
| TITANIUM DIOXIDE 4.5% | Sunscreen |
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
Inactive Ingredients
TRIETHYLHEXANOIN, POLYGLYCERYL-2 TRIISOSTEARATE, TRIISOSTEARIN, HYDROGENATED POLYISOBUTENE, POLYETHYLENE, PEG/PPG-36/41 DIMETHYL ETHER, MICROCRYSTALLINE WAX, STEARYL GLYCYRRHETINATE, GLUTATHIONE, ZINC MYRISTATE, THIOTAURINE, SODIUM ACETYLATED HYALURONATE, ALUMINUM HYDROXIDE, TOCOPHEROL, DIISOSTEARYL MALATE, SODIUM MAGNESIUM SILICATE, GLYCERIN, BHT, FRAGRANCE,
Other information
- protect this product in this container from excessive heat and direct sun.
Questions or comments?
Call toll free 1-800-906-7503
PRINCIPAL DISPLAY PANEL - 4g Cartridge Carton
SHI
SEIDO
SPF 35
Sun Protection
Lip Treatment
SUNSCREEN
4g NET WT. .14 OZ.
SHISEIDO AMERICA INC.