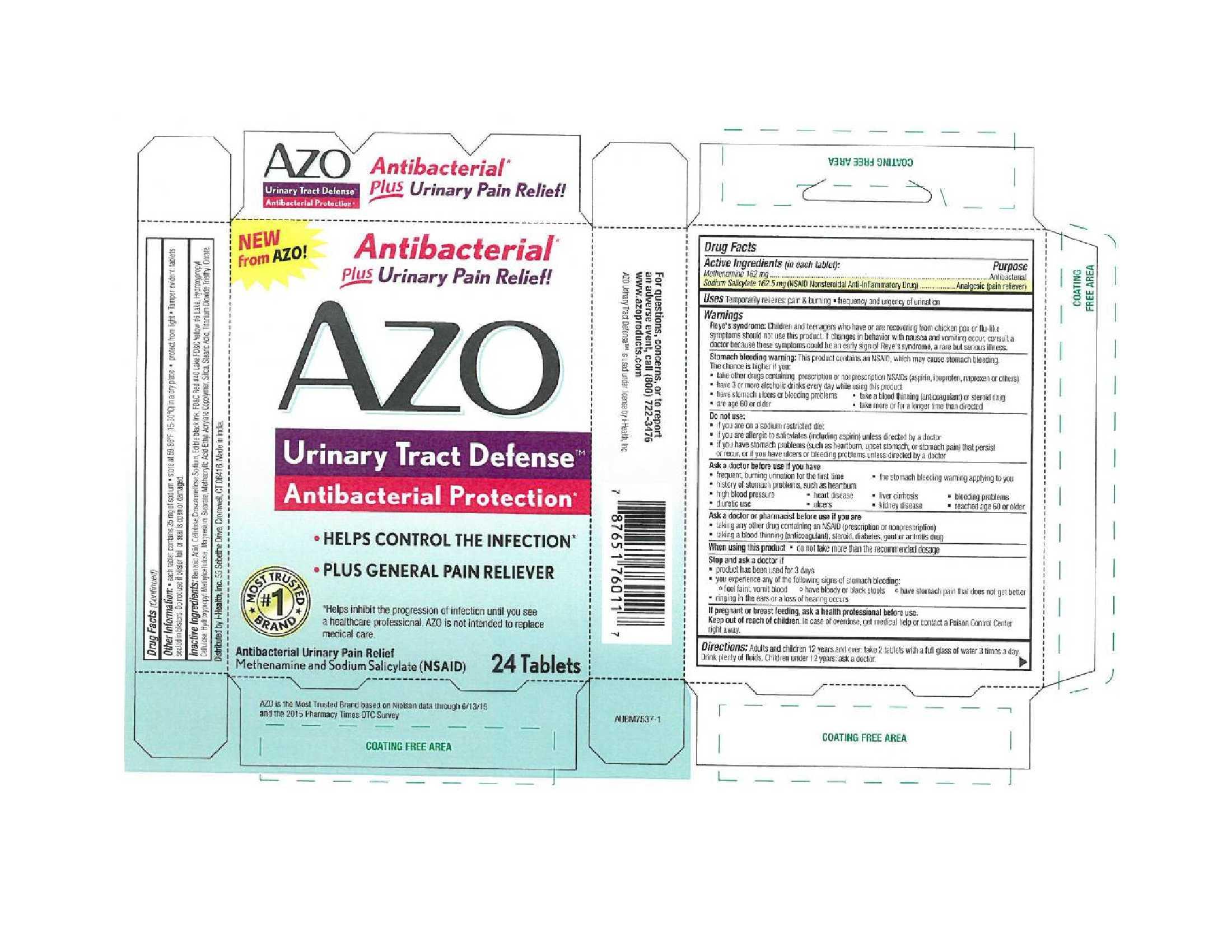
Drug Facts
Active Ingredients (in each tablet):
Methenamine 162 mg
Sodium Salicylate 162.5 (NSAID Nonsteroidal Anti-Inflammatory Drug)
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. If changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause stomach bleeding. The chance is higher if you:
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- have stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- are age 60 or older
- take more or for a longer time than directed
Do not use:
- if you are on a sodium restricted diet
- if you are allergic to salicylates (including aspirin) unless directed by a doctor
- if you have stomach problems (such as heartburn, upset stomach, or stomach pain) that persist or recur, or if you have ulcers or bleeding problems unless directed by a doctor
Ask a doctor before use if you have
- frequent, burning urination for the first time
- the stomach bleeding warning applying to you
- history of stomach problems, such as heartburn
- high blood pressure
- heart disease
- liver cirrhosis
- bleeding problems
- diuretic use
- ulcers
- kidney disease
- reached age 60 or older
Ask a doctor or pharmacist before use if you are
- taking any other drug containing an NSAID (prescription or nonprescription)
- taking a blood thinning (anticoagulant), steroid, diabetes, gout or arthritis drug
Directions: Adults and children 12 years and over: take 2 tablets with a full glass of water 3 times a day. Drink plenty of fluids. Children under 12 years: ask a doctor.
Other Information:
- each tablet contains 25 mg of sodium
- store at 59-86°F (15-30°C) in a dry place
- protect from light
- Tamper evidence tablets sealed in blisters. Do not use if blister foil or seal is open or damaged.
Inactive Ingredients: Benzoic Acid, Cellulose, Croscarmellose Sodium, Edible black ink, FD&C Red #40 Lake, FD&C Yellow #6 Lake, Hydroxypropyl Cellulose, Hydroxypropyl Methylcellulose, Magnesium Stearate, Methacrylic Acid - Ethyl Acrylate Copolymer, Silica, Stearic Acid, Titanium Dioxide, Triethyl Citrate