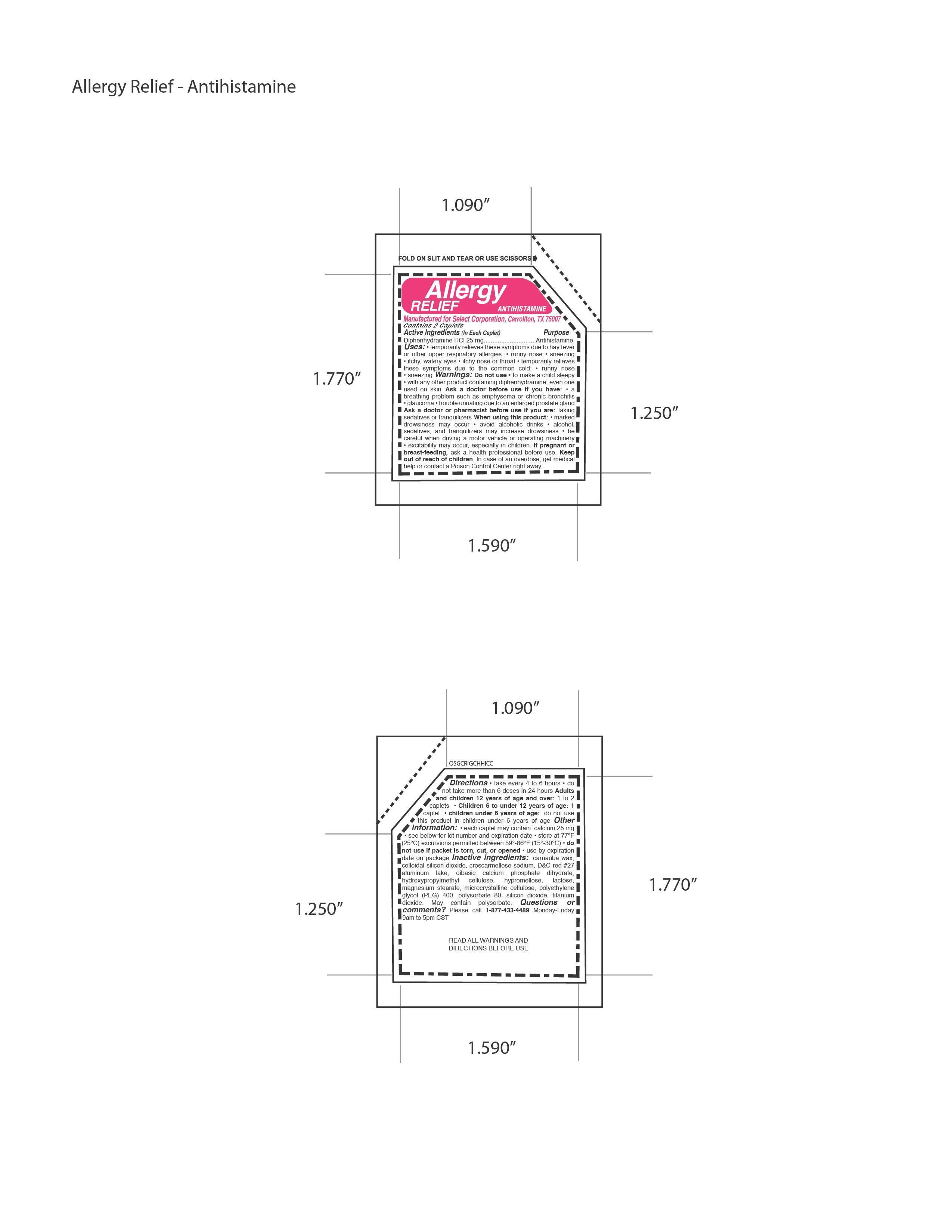
Directions • take every 4 to 6 hours • do
not take more than 6 doses in 24 hours Adults
and children 12 years of age and over: 1 to 2
caplets • Children 6 to under 12 years of age: 1
caplet • children under 6 years of age: do not use
this product in children under 6 years of age
Uses: • temporarily relieves these symptoms due to hay fever
or other upper respiratory allergies: • runny nose • sneezing
• itchy, watery eyes • itchy nose or throat • temporarily relieves
these symptoms due to the common cold: • runny nose
• sneezing
• with any other product containing diphenhydramine, even one
used on skin Ask a doctor before use if you have: • a
breathing problem such as emphysema or chronic bronchitis
• glaucoma • trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are: taking
sedatives or tranquilizers When using this product: • marked
drowsiness may occur • avoid alcoholic drinks • alcohol,
sedatives, and tranquilizers may increase drowsiness • be
careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children.
Inactive ingredients: carnauba wax,
colloidal silicon dioxide, croscarmellose sodium, DC red 27
aluminum lake, dibasic calcium phosphate dihydrate,
hydroxypropylmethyl cellulose, hypromellose, lactose,
magnesium stearate, microcrystalline cellulose, polyethylene
glycol (PEG) 400, polysorbate 80, silicon dioxide, titanium
dioxide