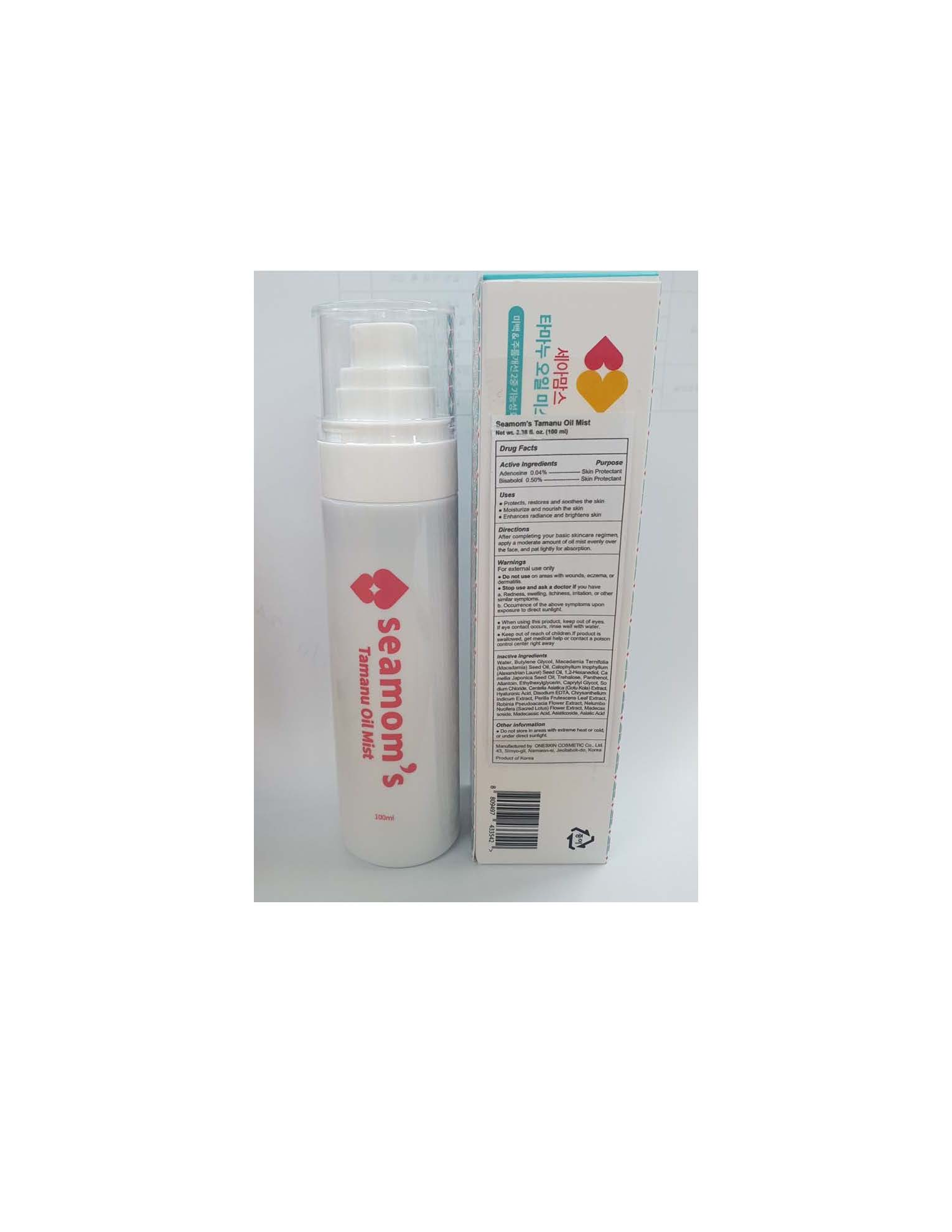
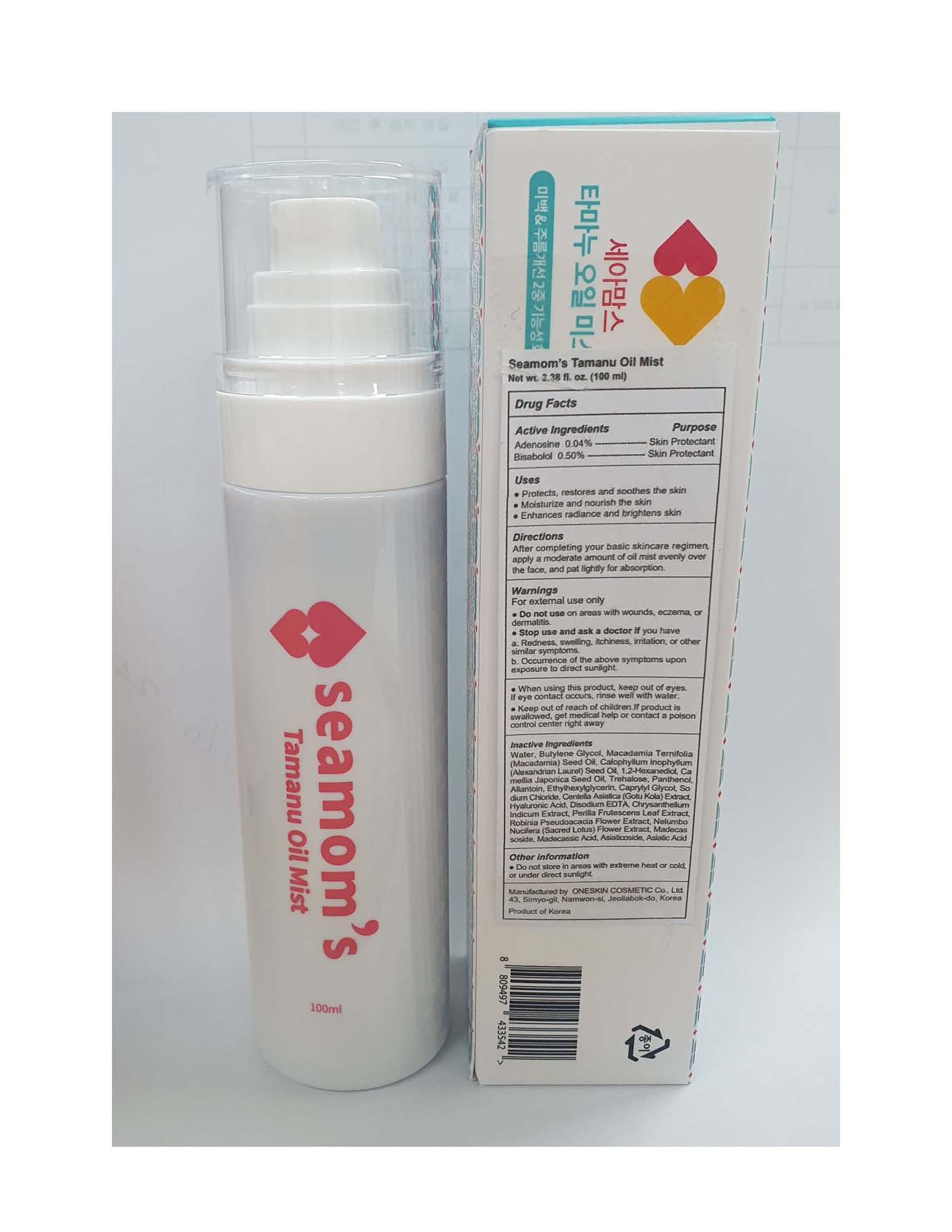
- Protects, restores and soothes the skin
- Moisturize and nourish the skin
- Enhances radiance and brightens skin
After completing your basic skincare regimen, apply a moderate amount of oil mist evenly over the face, and pat lightly for absorption.
For external use only
- Do not use on areas with wounds, eczema, or dermatitis.
- Stop use and ask a doctor if you have
a. Redness, swelling, itchiness, irritation, or other similar symptoms.
b. Occurrence of the above symptoms upon exposure to direct sunlight.
- When using this product, keep out of eyes. If eye contact occurs, rinse well with water.
- Keep out of reach of children. If product is swallowed, get medical help or contact a poison control center right away
Keep out of reach of children. If product is swallowed, get medical help or contact a poison control center right away
Water, Butylene Glycol, Macadamia Ternifolia (Macadamia) Seed Oil, Calophyllum Inophyllum (Alexandrian Laurel) Seed Oil, 1,2-Hexanediol, Camellia Japonica Seed Oil, Trehalose, Panthenol, Allantoin, Ethylhexylglycerin, Caprylyl Glycol, Sodium Chloride, Centella Asiatica (Gotu Kola) Extract, Hyaluronic Acid, Disodium EDTA, Chrysanthellum Indicum Extract, Perilla Frutescens Leaf Extract, Robinia Pseudoacacia Flower Extract, Nelumbo Nucifera (Sacred Lotus) Flower Extract, Madecassoside, Madecassic Acid, Asiaticoside, Asiatic Acid.