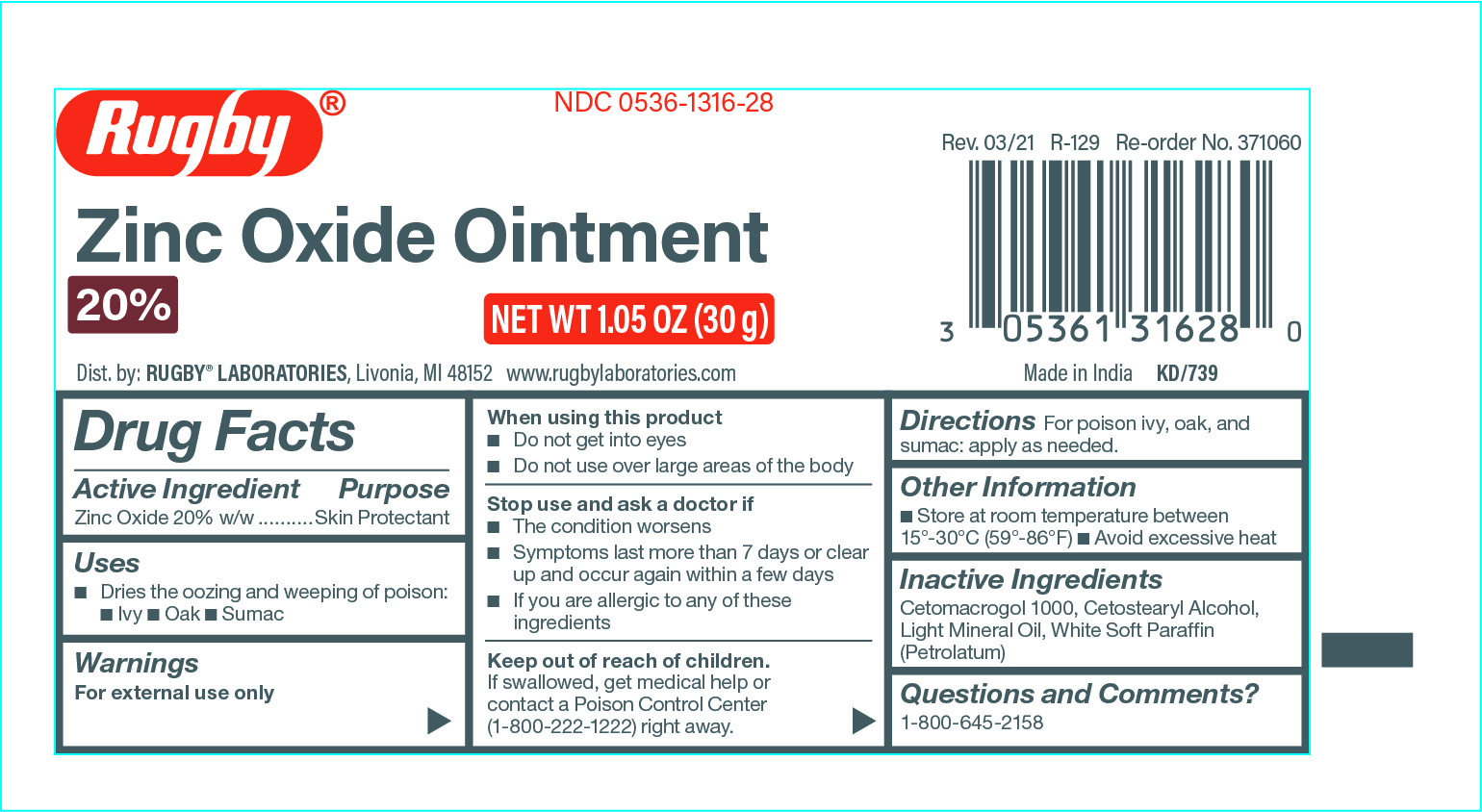
Active Ingredient
Zinc Oxide 20% w/w
Uses
• Dries the oozing and weeping of poison: • Ivy • Oak • Sumac
Warnings
For External Use Only
When using this product
• Do not get into eyes
• Do not use over large areas of the body
Stop use and ask a doctor if
• The condition worsens
• Symptoms last more than 7 days or clear up and occur again within a few days
• If you are allergic to any of these ingredients
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
For poison ivy, oak, and sumac: apply as needed.
Other Information
• Store at room temperature between 15°-30°C (59°-86°F)
• Avoid excessive heat
Inactive Ingredients
Cetomacrogol 1000, Cetostearyl Alcohol, Light Mineral Oil, White Soft Paraffin (Petrolatum)
Questions and Comments?
1-800-645-2158
Label


Label

Label