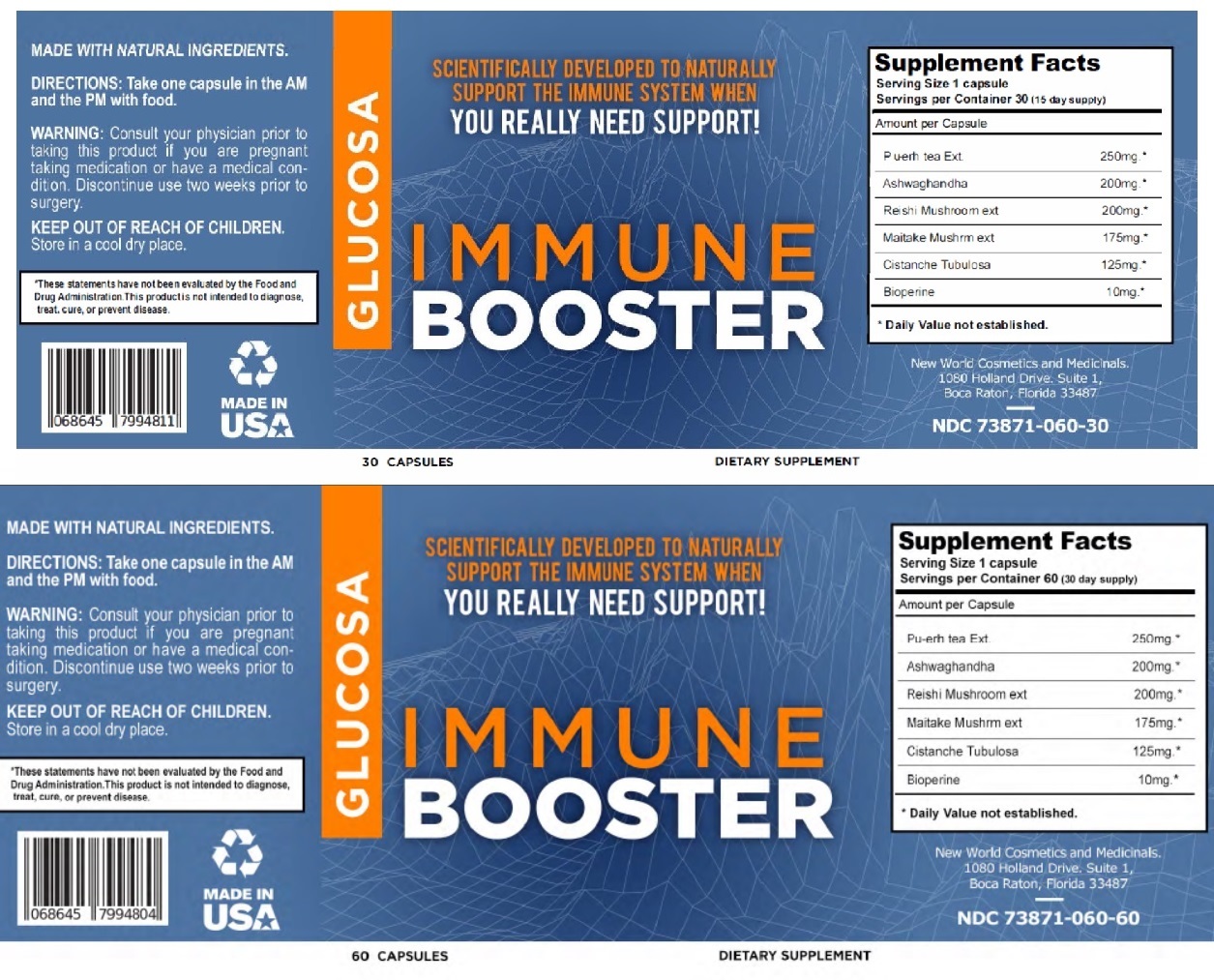
|
Supplement Facts
|
|
| Amount per Capsule | |
| Pu-erh tea Ext. | 250mg.* |
| Ashwagandha | 200mg.* |
| Reishi Mushroom ext | 200mg.* |
| Maitake Mushroom ext | 175mg.* |
| Cistanche Tubulosa | 125mg.* |
| Bioperine | 10mg.* |
| * Daily Value not established. | |
WARNING: Consult your physician prior to taking this product if you are pregnant taking medication or have a medical condition. Discontinue use two weeks prior to surgery.
SCIENTIFICALLY DEVELOPED TO NATURALLY SUPPORT THE IMMUNE SYSTEM WHEN YOU REALLY NEED SUPPORT!
MADE WITH NATURAL INGREDIENTS.
| *These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent disease. |
MADE IN USA
DIETARY SUPPLEMENT
New World Cosmetics and Medicinals.
1080 Holland Drive. Suite 1,
Boca Raton, Florida 33487