FULL PRESCRIBING INFORMATION
Reports of serious adverse events, including deaths in patients treated with OTFC have been reported. Deaths occurred as a result of improper patient selection (e.g., use in opioid non-tolerant patients) and/or improper dosing. The substitution of OTFC for any other fentanyl product may result in fatal overdose.
OTFC is indicated only for the management of breakthrough cancer pain in patients with malignancies who are already receiving and who are tolerant to around-the-clock opioid therapy for their underlying persistent cancer pain. Patients considered opioid tolerant are those who are taking around-the-clock medicine consisting of at least 60 mg of oral morphine daily, at least 25 mcg of transdermal fentanyl/hour, at least 30 mg of oral oxycodone daily, at least 8 mg oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
OTFC is not indicated for use in opioid non-tolerant patients including those with only as needed (PRN) prior exposure.
Life-threatening respiratory depression could occur at any dose in opioid non-tolerant patients. Deaths have occurred in opioid non-tolerant patients
OTFC is contraindicated in the management of acute or postoperative pain including headache/migraine.
When prescribing, do not convert patients on a mcg per mcg basis to OTFC from other fentanyl products.
When dispensing, do not substitute an OTFC prescription for other fentanyl products. Substantial differences exist in the pharmacokinetic profile of OTFC compared to other fentanyl products that result in clinically important differences in the extent of absorption of fentanyl. As a result of these differences, the substitution of OTFC for any other fentanyl product may result in fatal overdose.
Special care must be used when dosing OTFC. If the breakthrough pain episode is not relieved 15 minutes after completion of the OTFC unit, patients may take ONLY ONE additional dose using the same strength and then must wait at least 4 hours before taking another dose [see Dosage And Administration (2.2)].
OTFC contains fentanyl, an opioid agonist and a Schedule II controlled substance, with an abuse liability similar to other opioid analgesics. OTFC can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing OTFC in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse or diversion. Schedule II opioid substances which include morphine, oxycodone, hydromorphone, oxymorphone, and methadone have the highest potential for abuse and risk of fatal overdose due to respiratory depression
Patients and their caregivers must be instructed that OTFC contains a medicine in an amount which can be fatal to a child. Death has been reported in children who have accidentally ingested OTFC. All units must be kept out of the reach of children and opened units properly discarded. [see Warnings And Precautions (5.3), Patient Counseling Information (17.5, 17.6), and How Supplied/Storage And Handling (16.2)].
OTFC is intended to be used only in the care of cancer patients and only by oncologists and pain specialists who are knowledgeable of and skilled in the use of Schedule II opioids to treat cancer pain.
The concomitant use of OTFC with strong and moderate cytochrome P450 3A4 inhibitors may result in an increase in fentanyl plasma concentrations, and may cause potentially fatal respiratory depression [see Drug Interactions (7)].
1 INDICATIONS AND USAGE
Oral Transmucosal Fentanyl Citrate (OTFC) is indicated only for the management of breakthrough cancer pain in patients 16 and older with malignancies who are already receiving and who are tolerant to around-the-clock opioid therapy for their underlying persistent cancer pain. Patients considered opioid tolerant are those who are taking around-the-clock medicine consisting of at least 60 mg of oral morphine daily, at least 25 mcg of transdermal fentanyl/hour, at least 30 mg of oral oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid daily for a week or longer. Patients must remain on around-the-clock opioids when taking OTFC.
This product must not be used in opioid non-tolerant patients because life-threatening respiratory depression and death could occur at any dose in patients not on a chronic regimen of opioids. For this reason, OTFC is contraindicated in the management of acute or postoperative pain.
OTFC is intended to be used only in the care of cancer patients and only by oncologists and pain specialists who are knowledgeable of and skilled in the use of Schedule II opioids to treat cancer pain.
2 DOSAGE AND ADMINISTRATION
As with all opioids, the safety of patients using such products is dependent on health care professionals prescribing them in strict conformity with their approved labeling with respect to patient selection, dosing, and proper conditions for use.
2.1 Initial Dose
Individually titrate OTFC to a dose that provides adequate analgesia and minimizes side effects. The initial dose of OTFC to treat episodes of breakthrough cancer pain is always 200 mcg. The OTFC unit should be consumed over 15 minutes. Patients should be prescribed an initial titration supply of six 200 mcg OTFC units, thus limiting the number of units in the home during titration. Patients should use up all units before increasing to a higher dose to prevent confusion and possible overdose.
2.2 Dose Titration
From this initial dose, closely follow patients and change the dosage level until the patient reaches a dose that provides adequate analgesia using a single OTFC dosage unit per breakthrough cancer pain episode. If signs of excessive opioid effects appear before the unit is consumed, the dosage unit should be removed from the patient’s mouth immediately, disposed of properly, and subsequent doses should be decreased. Patients should record their use of OTFC over several episodes of breakthrough cancer pain and review their experience with their physicians to determine if a dosage adjustment is warranted.
In cases where the breakthrough pain episode is not relieved 15 minutes after completion of the OTFC unit (30 minutes after the start of the unit), patients may take ONLY ONE additional dose of the same strength for that episode. Thus, patients should take a maximum of two doses of OTFC for any breakthrough pain episode.
Patients must wait at least 4 hours before treating another episode of breakthrough pain with OTFC. To reduce the risk of overdosing during titration, patients should have only one strength of OTFC available at any one time.
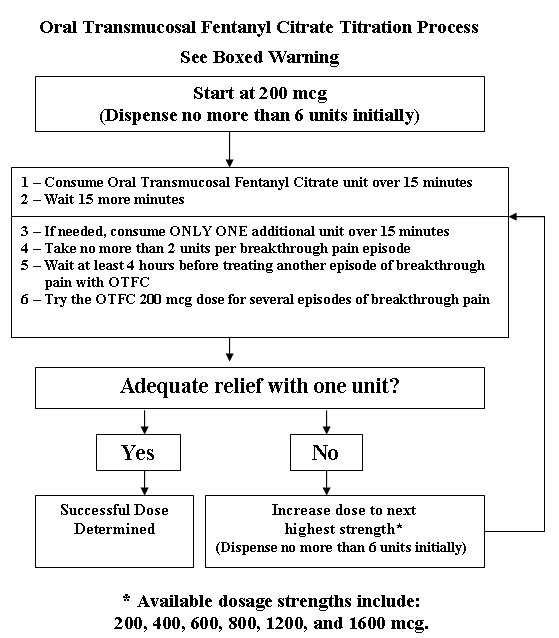
2.3 Maintenance Dosing
Once titrated to an effective dose, patients should generally use ONLY ONE OTFC unit of the appropriate strength per breakthrough pain episode.
On those occasions when the breakthrough pain episode is not relieved 15 minutes after completion of the OTFC unit, patient may take ONLY ONE additional dose using the same strength for that episode.
Patients MUST wait at least 4 hours before treating another episode of breakthrough pain with OTFC. Once a successful dose has been found (i.e., an average episode is treated with a single unit), patients should limit consumption to four or fewer units per day.
Dosage adjustment of OTFC may be required in some patients in order to continue to provide adequate relief of breakthrough pain.
Generally, the OTFC dose should be increased only when a single administration of the current dose fails to adequately treat the breakthrough pain episode for several consecutive episodes.
If the patient experiences greater than four breakthrough pain episodes per day, the dose of the maintenance (around-the-clock) opioid used for persistent pain should be re-evaluated.
2.4 Administration of OTFC
Open the blister package with scissors immediately prior to product use. The patient should place the OTFC unit in his or her mouth between the cheek and lower gum, occasionally moving the drug matrix from one side to the other using the handle. The OTFC unit should be sucked, not chewed. A unit dose of OTFC, if chewed and swallowed, might result in lower peak concentrations and lower bioavailability than when consumed as directed [see Clinical Pharmacology (12.3)].
The OTFC unit should be consumed over a 15-minute period. Longer or shorter consumption times may produce less efficacy than reported in OTFC clinical trials. If signs of excessive opioid effects appear before the unit is consumed, remove the drug matrix from the patient’s mouth immediately and decrease future doses.
3 DOSAGE FORMS AND STRENGTHS
Each dosage unit has white to off-white color and is a solid drug matrix on a handle. Each strength is marked on the individual solid drug matrix and the handle tag. OTFC is available in 200 mcg, 400 mcg, 600 mcg, 800 mcg, 1200 mcg and 1600 mcg strengths [see How Supplied/Storage And Handling (16.3)].
4 CONTRAINDICATIONS
OTFC is contraindicated in opioid non-tolerant patients. OTFC is contraindicated in the management of acute or postoperative pain including headache/migraine. Life-threatening respiratory depression and death could occur at any dose in opioid non-tolerant patients.
Patients considered opioid tolerant are those who are taking around-the-clock medicine consisting of at least 60 mg of oral morphine daily, at least 25 mcg of transdermal fentanyl/hour, at least 30 mg of oral oxycodone daily, at least 8 mg of oral hydromorphone daily, or an equianalgesic dose of another opioid daily for a week or longer.
OTFC is contraindicated in patients with known intolerance or hypersensitivity to any of its components or the drug fentanyl. Anaphylaxis and hypersensitivity have been reported in association with the use of OTFC.
5 WARNINGS AND PRECAUTIONS
See Boxed Warning - WARNING: IMPORTANCE OF PROPER PATIENT SELECTION, DOSING, and POTENTIAL FOR ABUSE
5.1 Important Information Regarding Prescribing and Dispensing
When prescribing, DO NOT convert a patient to OTFC from any other fentanyl product on a mcg per mcg basis as OTFC and other fentanyl products are not equivalent on a microgram per microgram basis.
OTFC is NOT a generic version of Fentora®. When dispensing, DO NOT substitute an OTFC prescription for a Fentora prescription under any circumstances. Fentora and OTFC are not equivalent. Substantial differences exist in the pharmacokinetic profile of OTFC compared to other fentanyl products including Fentora that result in clinically important differences in the rate and extent of absorption of fentanyl. As a result of these differences, the substitution of OTFC for any other fentanyl product may result in a fatal overdose.
There are no safe conversion directions available for patients on any other fentanyl products. (Note: This includes oral, transdermal, or parenteral formulations of fentanyl.) Therefore, for opioid tolerant patients, the initial dose of OTFC should always be 200 mcg. Each patient should be individually titrated to provide adequate analgesia while minimizing side effects [see Dosage And Administration (2.2)].
5.2 Respiratory Depression
As with all opioids, there is a risk of clinically significant respiratory depression in patients using OTFC. Accordingly, follow all patients for symptoms of respiratory depression. Respiratory depression may occur more readily when opioids are given in conjunction with other agents that depress respiration.
5.3 Patient/Caregiver Instructions
Patients and their caregivers must be instructed that OTFC contains a medicine in an amount which can be fatal to a child. Death has been reported in children who have accidentally ingested OTFC. Patients and their caregivers must be instructed to keep both used and unused dosage units out of the reach of children. While all units should be disposed of immediately after use, partially consumed units represent a special risk to children. In the event that a unit is not completely consumed it must be properly disposed as soon as possible [see How Supplied/Storage And Handling (16.1, 16.2), and Patient Counseling Information (17.1) and the Medication Guide].
Physicians and dispensing pharmacists must specifically question patients or caregivers about the presence of children in the home (on a full time or visiting basis) and counsel them regarding the dangers to children from inadvertent exposure.
OTFC could be fatal to individuals for whom it is not prescribed and for those who are not opioid-tolerant.
5.4 Additive CNS Depressant Effects
The concomitant use of OTFC with other CNS depressants, including other opioids, sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, skeletal muscle relaxants, sedating antihistamines, and alcoholic beverages may produce increased depressant effects (e.g., respiratory depression, hypotension, and profound sedation). Concomitant use with potent inhibitors of cytochrome P450 3A4 isoform (e.g., erythromycin, ketoconazole, and certain protease inhibitors) may increase fentanyl levels, resulting in increased depressant effects [see Drug Interactions (7)].
Patients on concomitant CNS depressants must be monitored for a change in opioid effects. Consideration should be given to adjusting the dose of OTFC if warranted.
5.5 Effects on Ability to Drive and Use Machines
Opioid analgesics impair the mental and/or physical ability required for the performance of potentially dangerous tasks (e.g., driving a car or operating machinery). Warn patients taking OTFC of these dangers and counsel them accordingly.
5.6 Chronic Pulmonary Disease
Because potent opioids can cause respiratory depression, titrate OTFC with caution in patients with chronic obstructive pulmonary disease or preexisting medical conditions predisposing them to respiratory depression. In such patients, even normal therapeutic doses of OTFC may further decrease respiratory drive to the point of respiratory failure.
5.7 Head Injuries and Increased Intracranial Pressure
Administer OTFC with extreme caution in patients who may be particularly susceptible to the intracranial effects of CO2 retention such as those with evidence of increased intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient with a head injury and should be used only if clinically warranted.
6 ADVERSE REACTIONS
Enter section text here
6.1 Clinical Studies Experience
The safety of OTFC (oral transmucosal fentanyl citrate) has been evaluated in 257 opioid-tolerant chronic cancer pain patients. The duration of OTFC use varied during the open-label study. Some patients were followed for over 21 months. The average duration of therapy in the open-label study was 129 days.
The adverse reactions seen with OTFC are typical opioid side effects. Frequently, these adverse reactions will cease or decrease in intensity with continued use of OTFC, as the patient is titrated to the proper dose. Expect opioid side effects and manage them accordingly.
The most serious adverse reactions associated with all opioids including OTFC are respiratory depression (potentially leading to apnea or respiratory arrest), circulatory depression, hypotension, and shock. Follow all patients for symptoms of respiratory depression.
Because the clinical trials of OTFC were designed to evaluate safety and efficacy in treating breakthrough cancer pain, all patients were also taking concomitant opioids, such as sustained-release morphine or transdermal fentanyl, for their persistent cancer pain. The adverse event data presented here reflect the actual percentage of patients experiencing each adverse effect among patients who received OTFC for breakthrough cancer pain along with a concomitant opioid for persistent cancer pain. There has been no attempt to correct for concomitant use of other opioids, duration of OTFC therapy, or cancer-related symptoms. Adverse reactions are included regardless of causality or severity.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Three short-term clinical trials with similar titration schemes were conducted in 257 patients with malignancy and breakthrough cancer pain. Data are available for 254 of these patients. The goal of titration in these trials was to find the dose of OTFC that provided adequate analgesia with acceptable side effects (successful dose). Patients were titrated from a low dose to a successful dose in a manner similar to current titration dosing guidelines. Table 1 lists, by dose groups, adverse reactions with an overall frequency of 1% or greater that occurred during titration and are commonly associated with opioid administration or are of particular clinical interest. The ability to assign a dose-response relationship to these adverse reactions is limited by the titration schemes used in these studies. Adverse reactions are listed in descending order of frequency within each body system.
| Dose Group | Percentage of Patients Reporting Event | |||||
|---|---|---|---|---|---|---|
| 200-600 mcg | 800-1400 mcg | 1600 mcg | >1600 mcg | Any Dose* | ||
| (n=230) | (n=138) | (n=54) | (n=41) | (n=254) | ||
| Body As A Whole | ||||||
|
| Asthenia | 6 | 4 | 0 | 7 | 9 |
|
| Headache | 3 | 4 | 6 | 5 | 6 |
|
| Accidental Injury | 1 | 1 | 4 | 0 | 2 |
| Digestive | ||||||
|
| Nausea | 14 | 15 | 11 | 22 | 23 |
|
| Vomiting | 7 | 6 | 6 | 15 | 12 |
|
| Constipation | 1 | 4 | 2 | 0 | 4 |
| Nervous | ||||||
|
| Dizziness | 10 | 16 | 6 | 15 | 17 |
|
| Somnolence | 9 | 9 | 11 | 20 | 17 |
|
| Confusion | 1 | 6 | 2 | 0 | 4 |
|
| Anxiety | 3 | 0 | 2 | 0 | 3 |
|
| Abnormal Gait | 0 | 1 | 4 | 0 | 2 |
|
| Dry Mouth | 1 | 1 | 2 | 0 | 2 |
|
| Nervousness | 1 | 1 | 0 | 0 | 2 |
|
| Vasodilatation | 2 | 0 | 2 | 0 | 2 |
|
| Hallucinations | 0 | 1 | 2 | 2 | 1 |
|
| Insomnia | 0 | 1 | 2 | 0 | 1 |
|
| Thinking Abnormal | 0 | 1 | 2 | 0 | 1 |
|
| Vertigo | 1 | 0 | 0 | 0 | 1 |
| Respiratory | ||||||
|
| Dyspnea | 2 | 3 | 6 | 5 | 4 |
| Skin | ||||||
|
| Pruritus | 1 | 0 | 0 | 5 | 2 |
|
| Rash | 1 | 1 | 0 | 2 | 2 |
|
| Sweating | 1 | 1 | 2 | 2 | 2 |
| Special Senses | ||||||
|
| Abnormal Vision | 1 | 0 | 2 | 0 | 2 |
* Any Dose = A patient who experienced the same adverse event at multiple doses was only counted once
The following adverse reactions not reflected in Table 1 occurred during titration with an overall frequency of 1% or greater and are listed in descending order of frequency within each body system.
Body as a Whole: Pain, fever, abdominal pain, chills, back pain, chest pain, infection
Cardiovascular: Migraine
Digestive: Diarrhea, dyspepsia, flatulence
Metabolic and Nutritional: Peripheral edema, dehydration
Nervous: Hypesthesia
Respiratory: Pharyngitis, cough increased
The following reactions occurred during titration with an overall frequency of less than 1% and are listed in descending order of frequency within each body system.
Body as a Whole: Flu syndrome, abscess, bone pain
Cardiovascular: Deep thrombophlebitis, hypertension, hypotension
Digestive: Anorexia, eructation, esophageal stenosis, fecal impaction, gum hemorrhage, mouth ulceration, oral moniliasis
Hemic and Lymphatic: Anemia, leukopenia
Metabolic and Nutritional: Edema, hypercalcemia, weight loss
Musculoskeletal: Myalgia, pathological fracture, myasthenia
Nervous: Abnormal dreams, urinary retention, agitation, amnesia, emotional lability, euphoria, incoordination, libido decreased, neuropathy, paresthesia, speech disorder
Respiratory: Hemoptysis, pleural effusion, rhinitis, asthma, hiccup, pneumonia, respiratory insufficiency, sputum increased
Skin and Appendages: Alopecia, exfoliative dermatitis
Special Senses: Taste perversion
Urogenital: Vaginal hemorrhage, dysuria, hematuria, urinary incontinence, urinary tract infection
A long-term extension study was conducted in 156 patients with malignancy and breakthrough cancer pain who were treated for an average of 129 days. Data are available for 152 of these patients. Table 2 lists by dose groups, adverse reactions with an overall frequency of 1% or greater that occurred during the long-term extension study and are commonly associated with opioid administration or are of particular clinical interest. Adverse reactions are listed in descending order of frequency within each body system.
| Dose Group | Percentage of Patients Reporting Event | |||||
|---|---|---|---|---|---|---|
| 200-600 mcg | 800-1400 mcg | 1600 mcg | >1600 mcg | Any Dose* | ||
| (n=98) | (n=83) | (n=53) | (n=27) | (n=152) | ||
| Body As A Whole | ||||||
|
| Asthenia | 25 | 30 | 17 | 15 | 38 |
|
| Headache | 12 | 17 | 13 | 4 | 20 |
|
| Accidental Injury | 4 | 6 | 4 | 7 | 9 |
|
| Hypertonia | 2 | 2 | 2 | 0 | 3 |
| Digestive | ||||||
|
| Nausea | 31 | 36 | 25 | 26 | 45 |
|
| Vomiting | 21 | 28 | 15 | 7 | 31 |
|
| Constipation | 14 | 11 | 13 | 4 | 20 |
|
| Intestinal Obstruction | 0 | 2 | 4 | 0 | 3 |
| Cardiovascular | ||||||
|
| Hypertension | 1 | 1 | 0 | 0 | 1 |
| Nervous | ||||||
|
| Dizziness | 12 | 10 | 9 | 0 | 16 |
|
| Anxiety | 9 | 8 | 8 | 7 | 15 |
|
| Somnolence | 8 | 13 | 8 | 7 | 15 |
|
| Confusion | 2 | 5 | 13 | 7 | 10 |
|
| Depression | 9 | 4 | 2 | 7 | 9 |
|
| Insomnia | 5 | 1 | 8 | 4 | 7 |
|
| Abnormal Gait | 5 | 1 | 0 | 0 | 4 |
|
| Dry Mouth | 3 | 1 | 2 | 4 | 4 |
|
| Nervousness | 2 | 2 | 0 | 4 | 3 |
|
| Stupor | 4 | 1 | 0 | 0 | 3 |
|
| Vasodilatation | 1 | 1 | 4 | 0 | 3 |
|
| Thinking Abnormal | 2 | 1 | 0 | 0 | 2 |
|
| Abnormal Dreams | 1 | 1 | 0 | 0 | 1 |
|
| Convulsion | 0 | 1 | 2 | 0 | 1 |
|
| Myoclonus | 0 | 0 | 4 | 0 | 1 |
|
| Tremor | 0 | 1 | 2 | 0 | 1 |
|
| Vertigo | 0 | 0 | 4 | 0 | 1 |
| Respiratory | ||||||
|
| Dyspnea | 15 | 16 | 8 | 7 | 22 |
| Skin | ||||||
|
| Rash | 3 | 5 | 8 | 4 | 8 |
|
| Sweating | 3 | 2 | 2 | 0 | 4 |
|
| Pruritus | 2 | 0 | 2 | 0 | 2 |
| Special Senses | ||||||
|
| Abnormal Vision | 2 | 2 | 0 | 0 | 3 |
| Urogenital | ||||||
|
| Urinary Retention | 1 | 2 | 0 | 0 | 2 |
The following reactions not reflected in Table 2 occurred with an overall frequency of 1% or greater in the long-term extension study and are listed in descending order of frequency within each body system.
Body as a Whole: Pain, fever, back pain, abdominal pain, chest pain, flu syndrome, chills, infection, abdomen enlarged, bone pain, ascites, sepsis, neck pain, viral infection, fungal infection, cachexia, cellulitis, malaise, pelvic pain
Cardiovascular: Deep thrombophlebitis, migraine, palpitation, vascular disorder
Digestive: Diarrhea, anorexia, dyspepsia, dysphagia, oral moniliasis, mouth ulceration, rectal disorder, stomatitis, flatulence, gastrointestinal hemorrhage, gingivitis, jaundice, periodontal abscess, eructation, glossitis, rectal hemorrhage
Hemic and Lymphatic: Anemia, leukopenia, thrombocytopenia, ecchymosis, lymphadenopathy, lymphedema, pancytopenia
Metabolic and Nutritional: Peripheral edema, edema, dehydration, weight loss, hyperglycemia, hypokalemia, hypercalcemia, hypomagnesemia
Musculoskeletal: Myalgia, pathological fracture, joint disorder, leg cramps, arthralgia, bone disorder
Nervous: Hypesthesia, paresthesia, hypokinesia, neuropathy, speech disorder
Respiratory: Cough increased, pharyngitis, pneumonia, rhinitis, sinusitis, bronchitis, epistaxis, asthma, hemoptysis, sputum increased
Skin and Appendages: Skin ulcer, alopecia
Special Senses: Tinnitus, conjunctivitis, ear disorder, taste perversion
Urogenital: Urinary tract infection, urinary incontinence, breast pain, dysuria, hematuria, scrotal edema, hydronephrosis, kidney failure, urinary urgency, urination impaired, breast neoplasm, vaginal hemorrhage, vaginitis
The following reactions occurred with a frequency of less than 1% in the long-term extension study and are listed in descending order of frequency within each body system.
Body as a Whole: Allergic reaction, cyst, face edema, flank pain, granuloma, bacterial infection, injection site pain, mucous membrane disorder, neck rigidity
Cardiovascular: Angina pectoris, hemorrhage, hypotension, peripheral vascular disorder, postural hypotension, tachycardia
Digestive: Cheilitis, esophagitis, fecal incontinence, gastroenteritis, gastrointestinal disorder, gum hemorrhage, hemorrhage of colon, hepatorenal syndrome, liver tenderness, tooth caries, tooth disorder
Hemic and Lymphatic: Bleeding time increased
Metabolic and Nutritional: Acidosis, generalized edema, hypocalcemia, hypoglycemia, hyponatremia, hypoproteinemia, thirst
Musculoskeletal: Arthritis, muscle atrophy, myopathy, synovitis, tendon disorder
Nervous: Acute brain syndrome, agitation, cerebral ischemia, facial paralysis, foot drop, hallucinations, hemiplegia, miosis, subdural hematoma
Respiratory: Hiccup, hyperventilation, lung disorder, pneumothorax, respiratory failure, voice alteration
Skin and Appendages: Herpes zoster, maculopapular rash, skin discoloration, urticaria, vesiculobullous rash
Special Senses: Ear pain, eye hemorrhage, lacrimation disorder, partial permanent deafness, partial transitory deafness
Urogenital: Kidney pain, nocturia, oliguria, polyuria, pyelonephritis
6.2 Post-Marketing Experience
Adverse reactions are reported voluntarily from a population of uncertain size, and, therefore, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of the reporting, or (3) strength of causal connection to OTFC.
The following adverse reactions have been identified during postapproval use of OTFC (which contains approximately 2 grams of sugar per unit):
Digestive: Dental decay of varying severity including dental caries, tooth loss, and gum line erosion.
General Disorders and Administration Site Conditions: Application site reactions including irritation, pain, and ulcer.
7 DRUG INTERACTIONS
Fentanyl is metabolized mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4); therefore potential interactions may occur when OTFC is given concurrently with agents that affect CYP3A4 activity. The concomitant use of OTFC with strong CYP3A4 inhibitors (e.g., ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, and nefazodone) or moderate CYP3A4 inhibitors (e.g., amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, and verapamil) may result in increased fentanyl plasma concentrations, potentially causing serious adverse drug effects including fatal respiratory depression. Patients receiving OTFC concomitantly with moderate or strong CYP3A4 inhibitors should be carefully monitored for an extended period of time. Dosage increase should be done conservatively.
Grapefruit and grapefruit juice decrease CYP3A4 activity, increasing blood concentrations of fentanyl, thus should be avoided.
Drugs that induce cytochrome P450 3A4 activity may have the opposite effects.
Concomitant use of OTFC with an MAO inhibitor, or within 14 days of discontinuation, is not recommended [see Warnings And Precautions (5.9)].
8 USE IN SPECIFIC POPULATIONS
Enter section text here
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. OTFC should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.No epidemiological studies of congenital anomalies in infants born to women treated with fentanyl during pregnancy have been reported.
Chronic maternal treatment with fentanyl during pregnancy has been associated with transient respiratory depression, behavioral changes, or seizures in newborn infants characteristic of neonatal abstinence syndrome.
In women treated acutely with intravenous or epidural fentanyl during labor, symptoms of neonatal respiratory or neurological depression were no more frequent than would be expected in infants of untreated mothers.
Transient neonatal muscular rigidity has been observed in infants whose mothers were treated with intravenous fentanyl.
Fentanyl is embryocidal in rats as evidenced by increased resorptions in pregnant rats at doses of 30 mcg/kg IV or 160 mcg/kg SC. Conversion to human equivalent doses indicates this is within the range of the human recommended dosing for OTFC.
Fentanyl citrate was not teratogenic when administered to pregnant animals. Published studies demonstrated that administration of fentanyl (10, 100, or 500 mcg/kg/day) to pregnant rats from day 7 to 21, of their 21 day gestation, via implanted microosmotic minipumps was not teratogenic (the high dose was approximately 3-times the human dose of 1600 mcg per pain episode on a mg/m2 basis). Intravenous administration of fentanyl (10 or 30 mcg/kg) to pregnant female rats from gestation day 6 to 18, was embryo or fetal toxic, and caused a slightly increased mean delivery time in the 30 mcg/kg/day group, but was not teratogenic.
8.2 Labor and Delivery
Fentanyl readily passes across the placenta to the fetus; therefore do not use OTFC during labor and delivery.
8.3 Nursing Mothers
Fentanyl is excreted in human milk; therefore, do not use OTFC in nursing women because of the possibility of sedation and/or respiratory depression in their infants. Symptoms of opioid withdrawal may occur in infants at the cessation of nursing by women using OTFC.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients below 16 years of age have not been established.
In a clinical study, 15 opioid-tolerant pediatric patients with breakthrough pain, ranging in age from 5 to 15 years, were treated with OTFC. The study was too small to allow conclusions on safety and efficacy in this patient population. Twelve of the fifteen opioid-tolerant children and adolescents aged 5 to 15 years in this study received OTFC at doses ranging from 200 mcg to 600 mcg. The mean (CV%; range) dose-normalized (to 200 mcg) Cmax and AUC0-8 values were 0.87 ng/mL (51%; 0.42-1.30) and 4.54 ng×h/mL (42%; 2.37-6.0), respectively, for children ages 5 to <11 years old (N = 3) and 0.68 ng/mL (72%; 0.15-1.44) and 8.38 (192%; 0.84-50.78), respectively, for children ages >11 to <16 y (N = 9).
8.5 Geriatric Use
Of the 257 patients in clinical studies of OTFC in breakthrough cancer pain, 61 (24%) were 65 years of age and older, while 15 (6%) were 75 years of age and older. Those patients over the age of 65 years were titrated to a mean dose that was about 200 mcg less than the mean dose titrated to by younger patients. No difference was noted in the safety profile of the group over 65 years of age as compared to younger patients in OTFC clinical trials.
Elderly patients have been shown to be more sensitive to the effects of fentanyl when administered intravenously, compared with the younger population. Therefore, exercise caution when individually titrating OTFC in elderly patients to provide adequate efficacy while minimizing risk.
8.6 Patients with Renal or Hepatic Impairment
Insufficient information exists to make recommendations regarding the use of OTFC in patients with impaired renal or hepatic function. Fentanyl is metabolized primarily via human cytochrome P450 3A4 isoenzyme system and mostly eliminated in urine. If the drug is used in these patients, it should be used with caution because of the hepatic metabolism and renal excretion of fentanyl.
9 DRUG ABUSE AND DEPENDENCE
Enter section text here
9.1 Controlled Substance
Fentanyl is a Schedule II controlled substance that can produce drug dependence of the morphine type. OTFC may be subject to misuse, abuse and addiction.
9.2 Abuse and Addiction
Manage the handling of OTFC to minimize the risk of diversion, including restriction of access and accounting procedures as appropriate to the clinical setting and as required by law [see How Supplied/Storage And Handling (16.1, 16.2)].
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. However, all patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common. "Drug-seeking" behavior is very common in addicts and drug abusers.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse for nonmedical purposes, often in combination with other psychoactive substances. Since OTFC may be diverted for non-medical use, careful record keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of patients, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
9.3 Dependence
Guide the administration of OTFC by the response of the patient. Physical dependence, per se, is not ordinarily a concern when one is treating a patient with chronic cancer pain, and fear of tolerance and physical dependence should not deter using doses that adequately relieve the pain.
Opioid analgesics may cause physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity, e.g., naloxone, nalmefene, or mixed agonist/antagonist analgesics (pentazocine, butorphanol, buprenorphine, nalbuphine).
Physical dependence usually does not occur to a clinically significant degree until after several weeks of continued opioid usage. Tolerance, in which increasingly larger doses are required in order to produce the same degree of analgesia, is initially manifested by a shortened duration of analgesic effect, and subsequently, by decreases in the intensity of analgesia.
10 OVERDOSAGE
Enter section text here
10.1 Clinical Presentation
The manifestations of OTFC overdosage are expected to be similar in nature to intravenous fentanyl and other opioids, and are an extension of its pharmacological actions with the most serious significant effect being respiratory depression [see Clinical Pharmacology (12.2)].
10.2 Immediate Management
Immediate management of opioid overdose includes removal of the OTFC unit, if still in the mouth, ensuring a patent airway, physical and verbal stimulation of the patient, and assessment of level of consciousness, ventilatory and circulatory status.
10.3 Treatment of Overdosage (Accidental Ingestion) in the Opioid NON-Tolerant Person
Provide ventilatory support, obtain intravenous access, and employ naloxone or other opioid antagonists as clinically indicated. The duration of respiratory depression following overdose may be longer than the effects of the opioid antagonist’s action (e.g., the half-life of naloxone ranges from 30 to 81 minutes) and repeated administration may be necessary. Consult the package insert of the individual opioid antagonist for details about such use.
10.4 Treatment of Overdose in Opioid-Tolerant Patients
Provide ventilatory support and obtain intravenous access as clinically indicated. Judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is associated with the risk of precipitating an acute withdrawal syndrome.
10.5 General Considerations for Overdose
Management of severe OTFC overdose includes: securing a patent airway, assisting or controlling ventilation, establishing intravenous access, and GI decontamination by lavage and/or activated charcoal, once the patient's airway is secure. In the presence of respiratory depression or apnea, assist or control ventilation, and administer oxygen as indicated.
Although muscle rigidity interfering with respiration has not been seen following the use of OTFC, this is possible with fentanyl and other opioids. If it occurs, manage it by using assisted or controlled ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking agent.
11 DESCRIPTION
OTFC (oral transmucosal fentanyl citrate) is a solid formulation of fentanyl citrate, a potent opioid analgesic, intended for oral transmucosal administration. OTFC is formulated as a white to off-white solid drug matrix on a handle that is fracture resistant (ABS plastic) under normal conditions when used as directed.
OTFC is designed to be dissolved slowly in the mouth to facilitate transmucosal absorption. The handle allows the OTFC unit to be removed from the mouth if signs of excessive opioid effects appear during administration.
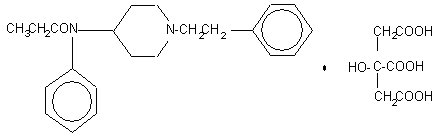
Active Ingredient: Fentanyl citrate, USP is N-(1-Phenethyl-4-piperidyl) propionanilide citrate (1:1). Fentanyl is a highly lipophilic compound (octanol-water partition coefficient at pH 7.4 is 816:1) that is freely soluble in organic solvents and sparingly soluble in water (1:40). The molecular weight of the free base is 336.5 (the citrate salt is 528.6). The pKa of the tertiary nitrogens are 7.3 and 8.4. The compound has the following structural formula:
Inactive Ingredients: Hydrated dextrates, citric acid, dibasic sodium phosphate, artificial berry flavor, magnesium stearate, and edible glue (modified food starch and confectioner's sugar).
12 CLINICAL PHARMACOLOGY
Enter section text here
12.1 Mechanism of Action
Fentanyl is a pure opioid agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as morphine, oxycodone, hydromorphone, codeine, and hydrocodone.
12.2 Pharmacodynamics
Pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, cough suppression, and analgesia. Like all pure opioid agonist analgesics, with increasing doses there is increasing analgesia, unlike with mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the analgesic effect with increasing doses. With pure opioid agonist analgesics, there is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by side effects, the more serious of which may include somnolence and respiratory depression.
AnalgesiaThe analgesic effects of fentanyl are related to the blood level of the drug, if proper allowance is made for the delay into and out of the CNS (a process with a 3- to 5-minute half-life).
In general, the effective concentration and the concentration at which toxicity occurs increase with increasing tolerance with any and all opioids. The rate of development of tolerance varies widely among individuals. As a result, the dose of OTFC should be individually titrated to achieve the desired effect [see Dosage And Administration (2.2)].
Central Nervous SystemThe precise mechanism of the analgesic action is unknown although fentanyl is known to be a mu-opioid receptor agonist. Specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug.
Fentanyl produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem to increases in carbon dioxide and to electrical stimulation.
Fentanyl depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia.
Fentanyl causes miosis even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings).
Gastrointestinal SystemFentanyl causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and in the duodenum. Digestion of food is delayed in the small intestine and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in gastric, biliary and pancreatic secretions, spasm of the sphincter of Oddi, and transient elevations in serum amylase.
Cardiovascular SystemFentanyl may produce release of histamine with or without associated peripheral vasodilation. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Endocrine SystemOpioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
Respiratory SystemAll opioid mu-receptor agonists, including fentanyl, produce dose-dependent respiratory depression. The risk of respiratory depression is less in patients receiving chronic opioid therapy who develop tolerance to respiratory depression and other opioid effects. During the titration phase of the clinical trials, somnolence, which may be a precursor to respiratory depression, did increase in patients who were treated with higher doses of OTFC. Peak respiratory depressive effects may be seen as early as 15 to 30 minutes from the start of oral transmucosal fentanyl citrate product administration and may persist for several hours.
Serious or fatal respiratory depression can occur even at recommended doses. Fentanyl depresses the cough reflex as a result of its CNS activity. Although not observed with oral transmucosal fentanyl products in clinical trials, fentanyl given rapidly by intravenous injection in large doses may interfere with respiration by causing rigidity in the muscles of respiration. Therefore, physicians and other healthcare providers should be aware of this potential complication [see Boxed Warning - Warning: Importance Of Proper Patient Selection, Dosing, and Potential for Abuse, Contraindications (4), Warnings And Precautions (5.2), Adverse Reactions (6), and Overdosage (10)].
12.3 Pharmacokinetics
AbsorbtionThe absorption pharmacokinetics of fentanyl from the oral transmucosal dosage form is a combination of an initial rapid absorption from the buccal mucosa and a more prolonged absorption of swallowed fentanyl from the GI tract. Both the blood fentanyl profile and the bioavailability of fentanyl will vary depending on the fraction of the dose that is absorbed through the oral mucosa and the fraction swallowed.
Absolute bioavailability, as determined by area under the concentration-time curve, of 15 mcg/kg in 12 adult males was 50% compared to intravenous fentanyl.
Normally, approximately 25% of the total dose of OTFC is rapidly absorbed from the buccal mucosa and becomes systemically available. The remaining 75% of the total dose is swallowed with the saliva and then is slowly absorbed from the GI tract. About 1/3 of this amount (25% of the total dose) escapes hepatic and intestinal first-pass elimination and becomes systemically available. Thus, the generally observed 50% bioavailability of OTFC is divided equally between rapid transmucosal and slower GI absorption. Therefore, a unit dose of OTFC, if chewed and swallowed, might result in lower peak concentrations and lower bioavailability than when consumed as directed.
Dose proportionality among four of the available strengths of OTFC (200, 400, 800, and 1600 mcg) has been demonstrated in a balanced crossover design in adult subjects (n=11). Mean serum fentanyl levels following these four doses of OTFC are shown in Figure 1. The curves for each dose level are similar in shape with increasing dose levels producing increasing serum fentanyl levels. Cmax and AUC0→∞ increased in a dose-dependent manner that is approximately proportional to the OTFC administered.
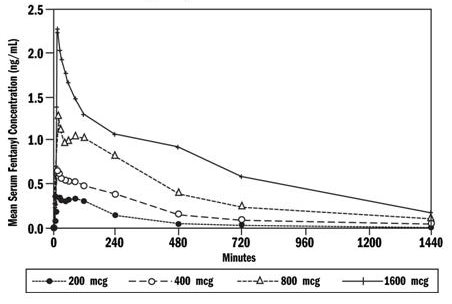
Figure 1. Mean Serum Fentanyl Concentration (ng/mL) in Adult Subjects Comparing 4 Doses of OTFC
The pharmacokinetic parameters of the four strengths of OTFC tested in the dose-proportionality study are shown in Table 3. The mean Cmax ranged from 0.39 - 2.51 ng/mL. The median time of maximum plasma concentration (Tmax) across these four doses of OTFC varied from 20 - 40 minutes (range of 20 - 480 minutes) as measured after the start of administration.
| Pharmacokinetic Parameter | 200 mcg | 400 mcg | 800 mcg | 1600 mcg |
|---|---|---|---|---|
| Tmax, minute median (range) | 40 (20-120) | 25 (20-240) | 25 (20-120) | 20 (20-480) |
| Cmax, ng/mL mean (%CV) |
0.39 (23) |
0.75 (33) |
1.55 (30) |
2.51 (23) |
| AUC0-1440, ng/mL minute mean (%CV) |
102 (65) |
243 (67) |
573 (64) |
1026 (67) |
| t1/2, minute mean (%CV) |
193 (48) |
386 (115) |
381 (55) |
358 (45) |
Distribution
Fentanyl is highly lipophilic. Animal data showed that following absorption, fentanyl is rapidly distributed to the brain, heart, lungs, kidneys and spleen followed by a slower redistribution to muscles and fat. The plasma protein binding of fentanyl is 80-85%. The main binding protein is alpha-1-acid glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of fentanyl increases with acidosis. The mean volume of distribution at steady state (Vss) was 4 L/kg.
MetabolismFentanyl is metabolized in the liver and in the intestinal mucosa to norfentanyl by cytochrome P450 3A4 isoform. Norfentanyl was not found to be pharmacologically active in animal studies [see Drug Interactions (7)].
EliminationFentanyl is primarily (more than 90%) eliminated by biotransformation to N-dealkylated and hydroxylated inactive metabolites. Less than 7% of the dose is excreted unchanged in the urine, and only about 1% is excreted unchanged in the feces. The metabolites are mainly excreted in the urine, while fecal excretion is less important. The total plasma clearance of fentanyl was 0.5 L/hr/kg (range 0.3 - 0.7 L/hr/kg). The terminal elimination half-life after OTFC administration is about 7 hours.
13 NONCLINICAL TOXICOLOGY
Enter section text here
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of fentanyl.
Fentanyl citrate was not mutagenic in the in vitro Ames reverse mutation assay in S. typhimurium or E. coli, or the mouse lymphoma mutagenesis assay, and was not clastogenic in the in vivo mouse micronucleus assay.
Fentanyl has been shown to impair fertility in rats at doses of 30 mcg/kg IV and 160 mcg/kg subcutaneously. Conversion to the human equivalent doses indicates that this is within the range of the human recommended dosing for OTFC.
14 CLINICAL STUDIES
OTFC was investigated in clinical trials involving 257 opioid tolerant adult cancer patients experiencing breakthrough cancer pain. Breakthrough cancer pain was defined as a transient flare of moderate-to-severe pain occurring in cancer patients experiencing persistent cancer pain otherwise controlled with maintenance doses of opioid medications including at least 60 mg morphine/day, 50 mcg transdermal fentanyl/hour, or an equianalgesic dose of another opioid for a week or longer.
In two dose titration studies 95 of 127 patients (75%) who were on stable doses of either long-acting oral opioids or transdermal fentanyl for their persistent cancer pain titrated to a successful dose of OTFC to treat their breakthrough cancer pain within the dose range offered (200, 400, 600, 800, 1200 and 1600 mcg). A "successful" dose was defined as a dose where one unit of OTFC could be used consistently for at least two consecutive days to treat breakthrough cancer pain without unacceptable side effects. In these studies 11% of patients withdrew due to adverse reactions and 14% withdrew due to other reasons.
The successful dose of OTFC for breakthrough cancer pain was not predicted from the daily maintenance dose of opioid used to manage the persistent cancer pain and is thus best determined by dose titration.
A double-blind placebo controlled crossover study was performed in cancer patients to evaluate the effectiveness of OTFC for the treatment of breakthrough cancer pain. Of 130 patients who entered the study 92 patients (71%) achieved a successful dose during the titration phase. The distribution of successful doses is shown in Table 4.
| OTFC Dose
| Total No. (%) (N=92) |
| 200 mcg | 13 (14) |
| 400 mcg | 19 (21) |
| 600 mcg | 14 (15) |
| 800 mcg | 18 (20) |
| 1200 mcg | 13 (14) |
| 1600 mcg | 15 (16) |
| __________ | ___________ |
| Mean ±SD | 789±468 mcg |
On average, patients over 65 years of age titrated to a mean dose that was about 200 mcg less than the mean dose to which younger adult patients were titrated.
OTFC was administered beginning at Time 0 minutes and produced more pain relief compared with placebo at 15, 30, 45, and 60 minutes as measured after the start of administration (see Figure 2). The differences were statistically significant.
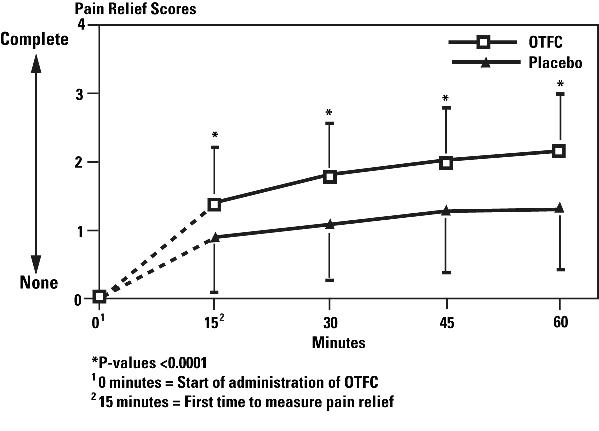
Figure 2. Pain Relief (PR) Scores (Mean±SD) During the Double-Blind Phase - All Patients with Evaluable Episodes on Both OTFC and Placebo (N=86)
16 HOW SUPPLIED/STORAGE AND HANDLING
Enter section text here
16.1 Storage and Handling
OTFC (oral transmucosal fentanyl citrate) is supplied in individually sealed child-resistant blister packages. The amount of fentanyl contained in OTFC can be fatal to a child. Patients and their caregivers must be instructed to keep OTFC out of the reach of children [see Boxed Warning - Warning: Importance Of Proper Patient Selection, Dosing, and Potential For Abuse, Warnings And Precautions (5), and Patient Counseling Information (17.1)].
Store at 20-25ºC (68-77ºF) with excursions permitted between 15° and 30°C (59° to 86°F) until ready to use. (See USP Controlled Room Temperature.) Protect OTFC from freezing and moisture. Do not use if the blister package has been opened.
16.2 Disposal of OTFC
Patients must be advised to dispose of any units remaining from a prescription as soon as they are no longer needed. While all units should be disposed of immediately after use, partially consumed units represent a special risk because they are no longer protected by the child resistant blister package, yet may contain enough medicine to be fatal to a child [see Patient Counseling Information (17.5)].
A temporary storage bottle is provided as part of the OTFC Child Safety Kit [see Patient Counseling Information (17.4)]. This container is to be used by patients or their caregivers in the event that a partially consumed unit cannot be disposed of promptly. Instructions for usage of this container are included in the Medication Guide.
Patients and members of their household must be advised to dispose of any units remaining from a prescription as soon as they are no longer needed. Instructions are included in Patient Counseling Information (17.6) and in the Medication Guide. If additional assistance is required, call Cephalon, Inc., at 1-800-896-5855.
16.3 How Supplied
OTFC is supplied in six dosage strengths. Each unit is individually wrapped in a child-resistant, protective blister package. These blister packages are packed 30 per shelf carton for use when patients have been titrated to the appropriate dose.
Each dosage unit has a white to off-white color. Each individual solid drug matrix is marked with "FENTANYL" and the strength of the unit ("200" ,"400", "600", "800" ,"1200", or "1600"). The dosage strength is also marked on the handle tag, the blister package and the carton. See blister package and carton for product information.
| Dosage Strength (fentanyl base) | Carton/Blister Package Color | NDC Number |
|---|---|---|
| 200 mcg | Gray | NDC 54868-6025-0 |
| 400 mcg | Blue | NDC 54868-5975-0 |
| 600 mcg | Orange | NDC 54868-6026-0 |
| 800 mcg | Purple | NDC 54868-6027-0 |
| 1200 mcg | Green | NDC 54868-6037-0 |
Note: Colors are a secondary aid in product identification. Please be sure to confirm the printed dosage before dispensing.
17 PATIENT COUNSELING INFORMATION
See the Medication Guide for specific patient instructions.
17.1 Patient/Caregiver Instructions
- Patients and their caregivers must be instructed that children exposed to OTFC are at high risk of FATAL RESPIRATORY DEPRESSION. Patients and their caregivers must be instructed to keep OTFC out of the reach of children [See How Supplied/Storage And Handling (16.1), Warnings And Precautions (5.2 and 5.3) and Medication Guide for specific patient instructions.]
- Provide patients and their caregivers with a Medication Guide each time OTFC is dispensed because new information may be available
- Instruct patients and their caregivers to keep both used and unused dosage units out of the reach of children. Partially consumed units represent a special risk to children. In the event that a unit is not completely consumed it must be properly disposed as soon as possible [see How Supplied/Storage And Handling (16.1), Warnings And Precautions (5.3), and Patient Counseling Information (17.5)]
- Instruct patients not to take OTFC for acute pain, postoperative pain, pain from injuries, headache, migraine or any other short-term pain, even if they have taken other opioid analgesics for these conditions
- Instruct patients on the meaning of opioid tolerance and that OTFC is only to be used as a supplemental pain medication for patients with pain requiring around-the-clock opioids, who have developed tolerance to the opioid medication, and who need additional opioid treatment of breakthrough pain episodes
- Instruct patients that, if they are not taking an opioid medication on a scheduled basis (around-the-clock), they should not take OTFC
- Instruct patients that, if the breakthrough pain episode is not relieved 15 minutes after finishing the OTFC unit, they may take ONLY ONE ADDITIONAL UNIT OF OTFC USING THE SAME STRENGTH FOR THAT EPISODE. Thus, patients should take no more than two units of OTFC for any breakthrough pain episode.
- Instruct patients that they MUST wait at least 4 hours before treating another episode of breakthrough pain with OTFC
- Instruct patients NOT to share OTFC and that sharing OTFC with anyone else could result in the other individual's death due to overdose
- Make patients aware that OTFC contains fentanyl which is a strong pain medication similar to hydromorphone, methadone, morphine, oxycodone, and oxymorphone
- Instruct patients that the active ingredient in OTFC, fentanyl, is a drug that some people abuse. OTFC should be taken only by the patient it was prescribed for, and it should be protected from theft or misuse in the work or home environment
- Caution patients to talk to their doctor if breakthrough pain is not alleviated or worsens after taking OTFC
- Instruct patients to use OTFC exactly as prescribed by their doctor and not to take OTFC more often than prescribed
- Caution patients that OTFC can affect a person's ability to perform activities that require a high level of attention (such as driving or using heavy machinery). Warn patients taking OTFC of these dangers and counsel them accordingly
- Warn patients to not combine OTFC with alcohol, sleep aids, or tranquilizers except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death
- Inform female patients that if they become pregnant or plan to become pregnant during treatment with OTFC, they should ask their doctor about the effects that OTFC (or any medicine) may have on them and their unborn children
17.2 Dental Care
Because each OTFC unit contains approximately 2 grams of sugar (hydrated dextrates), frequent consumption may increase the risk of dental decay. The occurrence of dry mouth associated with the use of opioid medications (such as fentanyl) may add to this risk.
Post-marketing reports of dental decay have been received in patients taking OTFC [see Adverse Reactions (6.2)]. In some of these patients, dental decay occurred despite reported routine oral hygiene. As dental decay in cancer patients may be multi-factorial, patients using OTFC should consult their dentist to ensure appropriate oral hygiene.
17.3 Diabetic Patients
Advise diabetic patients that OTFC contains approximately 2 grams of sugar per unit.
17.4 OTFC Child Safety Kit
Provide patients and their caregivers with an OTFC Child Safety Kit, which contains educational materials and safe interim storage containers to help patients store OTFC and other medicines out of the reach of children. To obtain a supply of Child Safety Kits, health care professionals can call Cephalon, Inc., at 1-800-896-5855.
17.5 Disposal of Used OTFC Units
Patients must be instructed to dispose of completely used and partially used OTFC units.
- After consumption of the unit is complete and the matrix is totally dissolved, throw away the handle in a trash container that is out of the reach of children.
- If any of the drug matrix remains on the handle, place the handle under hot running tap water until all of the drug matrix is dissolved, and then dispose of the handle in a place that is out of the reach of children.
- Dispose of handles in the child-resistant container (as described in steps 1 and 2) at least once a day.
If the patient does not entirely consume the unit and the remaining drug cannot be immediately dissolved under hot running water, the patient or caregiver must temporarily store the OTFC unit in the specially provided child-resistant container out of the reach of children until proper disposal is possible.
17.6 Disposal of Unopened OTFC Units When No Longer Needed
Patients and members of their household must be advised to dispose of any unopened units remaining from a prescription as soon as they are no longer needed.
To dispose of the unused OTFC units:
- Remove the OTFC unit from its blister package using scissors, and hold the OTFC by its handle over the toilet bowl.
- Using wire-cutting pliers cut off the drug matrix end so that it falls into the toilet.
- Dispose of the handle in a place that is out of the reach of children.
- Repeat steps 1, 2, and 3 for each OTFC unit. Flush the toilet twice after 5 units have been cut and deposited into the toilet.
Do not flush the entire OTFC units, OTFC handles, blister packages, or cartons down the toilet. Dispose of the handle where children cannot reach it [see How Supplied/Storage And Handling (16.1)].
Detailed instructions for the proper storage, administration, disposal, and important instructions for managing an overdose of OTFC are provided in the OTFC Medication Guide. Encourage patients to read this information in its entirety and giv then an opportunity to have their questions answered.
In the event that a caregiver requires additional assistance in disposing of excess unusable units that remain in the home after a patient has expired, instruct them to call the toll-free number for Cephalon, Inc., (1-800-896-5855) or seek assistance from their local DEA office.
Oral Transmucosal Fentanyl Citrate CII (OTFC)
200 mcg, 400 mcg, 600 mcg, 800 mcg, 1200 mcg, 1600 mcg
 WARNING:
WARNING:
- Do not use Oral Transmucosal Fentanyl Citrate (OTFC) unless you are regularly using other opioid pain medicines around-the-clock for your constant cancer pain and your body is used to these medicines.
- You MUST keep OTFC in a safe place out of the reach of children. Accidental ingestion by a child is a medical emergency and can result in death. Death has been reported in children who have accidentally taken OTFC. If a child accidentally takes OTFC, get emergency help right away.
Read the Medication Guide that comes with Oral Transmucosal Fentanyl Citrate (OTFC) before you start taking it and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or your treatment. Share this important information with members of your household.
What is the most important information I should know about Oral Transmucosal Fentanyl Citrate (OTFC)?
-
OTFC can cause life threatening breathing problems which
can lead to death:
- if you are not regularly using other opioid pain medicines around-the-clock for your constant cancer pain and your body is not used to these medicines. This means that you are not opioid tolerant.
- if you do not use it exactly as prescribed by your doctor.
- Your doctor will prescribe a starting dose of OTFC that is different than other fentanyl containing medicines you may have been taking. Do not substitute OTFC for other fentanyl medicines without talking with your doctor.
- Use no more than 2 units of OTFC per episode of breakthrough cancer pain. You must wait at least 4 hours before using OTFC again for another episode of breakthrough cancer pain.
What is Oral Transmucosal Fentanyl Citrate (OTFC)?
- OTFC is a prescription medicine that contains the medicine fentanyl. OTFC is a federally controlled substance (CII) because it is a strong opioid pain medicine that can be abused by people who abuse prescription medicines or street drugs.
- OTFC is to be used only to treat breakthrough pain in adult patients with cancer (16 years of age and older) who are already using other opioid pain medicines for their constant (around-the-clock) cancer pain. OTFC is started only after you have been taking other opioid pain medicines and your body has gotten used to them (you are opioid tolerant). DO NOT USE OTFC if you are not opioid tolerant. Please talk with your doctor about whether or not you are opioid tolerant.
- You must stay under your doctor’s care while taking OTFC.
- OTFC must not be used for the treatment of short-term pain from injuries, surgery, or headaches, including migraines.
- Prevent theft and misuse. Keep OTFC in a safe place to protect it from being stolen since it can be a target for people who abuse narcotic medicines or street drugs.
- Never give OTFC to anyone else, even if they have the same symptoms you have. It may harm them and even cause death.
- Selling or giving away this medicine is against the law.
Who should not take Oral Transmucosal Fentanyl Citrate
(OTFC)?
Do Not Take OTFC:
- if you are not already taking other opioid pain medicines around-the-clock for your constant cancer pain.
- for the treatment of short-term pain from injuries, surgery, or headaches, including migraines, or for pain that will go away in a few days, such as pain from doctor or dentist visits, or any short-lasting pain.
- if you are allergic to anything in OTFC. The active ingredient in OTFC is fentanyl. See the end of this Medication Guide for a complete list of ingredients in OTFC.
What should I tell my doctor before I start taking Oral Transmucosal Fentanyl Citrate (OTFC)?
Tell your doctor about all of your medical and mental problems, especially the ones listed below:
- Trouble breathing or lung problems such as asthma, wheezing, or shortness of breath
- A head injury or brain problem
- Liver or kidney problems
- Seizures (convulsions or fits)
- Slow heart rate or other heart problems
- Low blood pressure
- Mental problems including major depression or hallucinations (seeing or hearing things that are not there)
- A past or present drinking problem or alcoholism, or a family history of this problem
- A past or present drug abuse or addiction problem, or a family history of this problem
- If you are diabetic. Each OTFC unit contains about ½ teaspoon (2 grams) of sugar.
Tell your doctor if you are:
- pregnant or planning to become pregnant. OTFC may harm your unborn baby.
- breast feeding. Fentanyl passes through your breast milk and it can cause serious harm to your baby. You should not use OTFC while breast feeding.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal and dietary supplements. Some medicines may cause serious or life-threatening medical problems when taken with OTFC. Sometimes, the doses of certain medicines and OTFC need to be changed if used together.
Do not take any medicine while using OTFC until you have talked to your doctor. Your doctor will tell you if it is safe to take other medicines while you are using OTFC. Be especially careful about other medicines that make you sleepy such as other pain medicines, anti-depressant medicines, sleeping pills, anxiety medicines, antihistamines, or tranquilizers.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist.
How should I use Oral Transmucosal Fentanyl Citrate (OTFC)?
- Use OTFC exactly as prescribed by your doctor. Do not take OTFC more often than prescribed. Talk to your doctor about your pain. Your doctor can decide if your dose of OTFC needs to be changed.
- Each unit of OTFC is sealed in its own blister package.
- Do not open the blister package until you are ready to use OTFC.
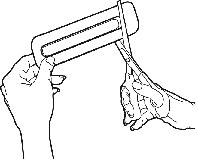
- When you are ready to use OTFC, cut open the package using scissors and remove the OTFC unit. The end of the unit marked with "FENTANYL" and the strength of the unit ("200", "400", "600", "800", "1200", or "1600") is the end that is to be placed in your mouth as described below.

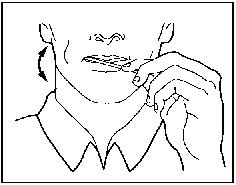
- Place OTFC in your mouth between your cheeks and gums and actively suck on the medicine.
- Move OTFC around in your mouth, especially along your cheeks.
- Twirl the handle often.
- Finish the OTFC unit completely in 15 minutes to get the most relief. If you finish OTFC too quickly, you will swallow more of the medicine and get less relief.
- Do not bite or chew OTFC. You will get less relief for your breakthrough pain.
- You may drink some water before using OTFC but you should not drink or eat anything while using OTFC.
- If you begin to feel dizzy, sick to your stomach, or very sleepy before OTFC is completely dissolved, remove OTFC from your mouth. Dispose of OTFC right away or put it in the temporary storage bottle in the Child Safety Kit for later disposal.
- Use 1 unit of OTFC for an episode of breakthrough cancer pain. Consume the unit over 15 minutes.
- If your breakthrough cancer pain is not relieved 15 minutes after you finished the OTFC unit, use ONLY 1 more dose of OTFC at this time.
- Wait at least 4 more hours before using OTFC again for another episode of breakthrough cancer pain.
- Remember, you must continue taking your regularly-used around-the-clock opioid medicine while taking OTFC.
- Limit the use of OTFC to four or fewer units per day.
- Talk to your doctor if your dose of OTFC does not relieve your breakthrough cancer pain. Your doctor will decide if your dose of OTFC needs to be changed.
- Talk to your doctor if you have more than 4 episodes of breakthrough cancer pain per day. The dose of your around-the-clock opioid pain medicine may need to be adjusted.
- If you take too much OTFC or overdose, call 911 or your local emergency number for help.
How should I dispose of Oral Transmucosal Fentanyl Citrate
(OTFC) after use?
Partially used OTFC units may contain enough medicine to be harmful or fatal to a child or other adults who have not been prescribed OTFC. You must properly dispose of the OTFC handle right away after use even if there is little or no medicine left on it. Please follow these directions to dispose of the handle:
- Once you have finished the OTFC unit and the medicine is totally gone, throw the handle away in a place that is out of the reach of children.
- If any medicine remains on the handle after you have finished, place the
handle under hot running water until the medicine is gone, and then throw the
handle away out of the reach of children and pets.
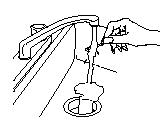
- If you did not finish the entire OTFC unit and you cannot dissolve the medicine under hot running water right away, put the OTFC in the temporary storage bottle that you received in the OTFC Child Safety Kit for safe keeping. Push the OTFC unit into the opening on the top until it falls completely into the bottle. Never leave unused or partially used OTFC units where children or pets can get to them.
- Dispose of the handles in the temporary storage bottle as soon as you can by following the directions in steps 1 and 2. You must dispose of all handles in the temporary storage bottle at least once a day.
Do not flush entire unused OTFC units, OTFC handles, or blister packages down the toilet.
What should I avoid while taking Oral Transmucosal Fentanyl Citrate
(OTFC)?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how OTFC affects how alert you are. OTFC can make you sleepy. Ask your doctor when it is okay to do these activities.
- Do not drink alcohol while using OTFC. It can increase your chance of getting dangerous side effects.
- Do not take any medicine while using OTFC until you have talked to your doctor. Your doctor will tell you if it is safe to take other medicines while you are using OTFC. Be especially careful about medicines that make you sleepy such as other pain medicines, anti-depressant medicines, sleeping pills, anxiety medicines, antihistamines, or tranquilizers.
What are the possible or reasonably likely SIDE
EFFECTS of Oral
Transmucosal Fentanyl Citrate (OTFC)?
- OTFC can cause serious breathing problems that can become life-threatening, especially if used the wrong way. See “What is the most important information I should know about OFTC?"
-
Call your doctor or get emergency medical help right away
if you:
- have trouble breathing
- have extreme drowsiness with slowed breathing
- have slow shallow breathing (little chest movement with breathing)
- feel faint, very dizzy, confused, or have unusual symptoms
These can be symptoms that you have taken too much (overdose) OTFC or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away. Do not take another dose of OTFC.
- OTFC can cause your blood pressure to drop. This can make you feel dizzy if you get up too fast from sitting or lying down.
- OTFC can cause physical dependence. Do not stop taking OTFC or any other opioid without talking to your doctor. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Physical dependency is not the same as drug addiction.
- There is a chance of abuse or addiction with OTFC. The chance is higher if you are or have been addicted to or abused other medications, street drugs, or alcohol, or if you have a history of mental problems.
The most common side effects of OTFC are nausea, vomiting, dizziness and sleepiness. Other side effects include headache, low energy and constipation. Constipation (not often enough or hard bowel movements) is a very common side effect of pain medicines (opioids) including OTFC and is unlikely to go away without treatment. Talk to your doctor about dietary changes, and the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking OTFC.
OTFC contains sugar. Cavities and tooth decay have occurred in patients taking OTFC. When taking OTFC, you should talk to your dentist about proper care of your teeth.
Talk to your doctor about any side effects that bother you or that do not go away.
These are not all the possible side effects of OTFC. For a complete list, ask your doctor.Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
How should I store Oral Transmucosal Fentanyl Citrate
(OTFC)?
- Keep OTFC in a safe place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes OTFC, get emergency help right away.
- OTFC is supplied in single sealed child-resistant blister packages. Store OTFC at room temperature, 59° to 86°F (15° to 30°C) until ready to use.
- Always keep OTFC in a secure place to protect from theft.
How should I dispose of unopened Oral Transmucosal Fentanyl Citrate (OTFC) units when they are no
longer needed?
- Dispose of any unopened OTFC units remaining from a prescription as soon as they are no longer needed.
- If you are no longer using OTFC or if you have unused OTFC in your home,
please follow these steps to dispose of the OTFC as soon as possible.
- Remove all OTFC from the locked storage space.
- Remove one OTFC unit from its blister package using scissors, and hold the OTFC by its handle over the toilet bowl.
- Using wire-cutting pliers, cut the medicine end off so that it falls into the toilet.
- Throw the handle away in a place that is out of the reach of children.
- Repeat steps 2, 3, and 4 for each OTFC.
- Flush the toilet twice after 5 OTFC units have been cut. Do not flush more than 5 OTFC units at a time.
- Do not flush entire unused OTFC units, OTFC handles, or blister packages down the toilet.
If you need help with disposal of OTFC, call Cephalon, Inc., at 1-800-896-5855.
General Information About the Safe and Effective Use of Oral Transmucosal Fentanyl Citrate (OTFC)
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Use OTFC only for the purpose for which it was prescribed.
Do not give OTFC to other people, even if they have the same symptoms you have.
OTFC can harm other people and even cause death.
Sharing OTFC is against the law
This Medication Guide summarizes the most important information about OTFC. If you would like more information, talk with your doctor. You can also ask your pharmacist or doctor for information about OTFC that is written for healthcare professionals. You can also call Cephalon, Inc., at 1-800-896-5855.
What are the ingredients of Oral Transmucosal Fentanyl Citrate (OTFC)?
Active Ingredient: fentanyl citrate.
Inactive Ingredients: Sugar, citric acid, dibasic sodium phosphate, artificial berry flavor, magnesium stearate, modified food starch, and confectioner's sugar.
How do I use the Oral Transmucosal Fentanyl
Citrate (OTFC) Child Safety Kit?
- You can use the OTFC Child Safety Kit to help you store OTFC and your other medicines out of the reach of children. It is very important that you use the items in the OTFC Child Safety Kit to protect the children in your home.
- If you were not offered a Child Safety Kit when you received your medicine, call Cephalon, Inc., at 1-800-896-5855 to request one.
The OTFC Child Safety Kit contains important information on the safe storage and handling of OTFC. The kit contents include:
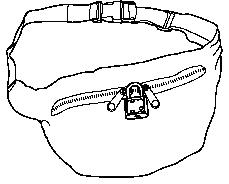
- A child-resistant lock for you to secure the storage space where you keep OTFC and any other medicines at home.
- A portable locking pouch for you to keep a small
supply of OTFC nearby for your immediate use. The rest of your OTFC must be kept
in the locked storage space.
- Keep this pouch secured with its lock and keep it out of the reach and sight of children.
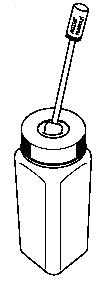
- A child-resistant temporary storage bottle.
- If for some reason you cannot finish the entire OTFC unit and cannot
immediately dissolve the medicine under hot tap water, immediately put the OTFC
unit in the temporary storage bottle for safe keeping.
- Push the OTFC unit into the opening on the top until it falls completely into the bottle. You must properly dispose of the OTFC unit as soon as you can.
- See “How should I dispose of unopened OTFC units when they are no longer needed?” for proper disposal of OTFC.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Rx Only
Manufactured by:
Anesta Corp., Salt Lake City, UT 84116, USA
Reference Number: 00010677.05
Printed in USA




