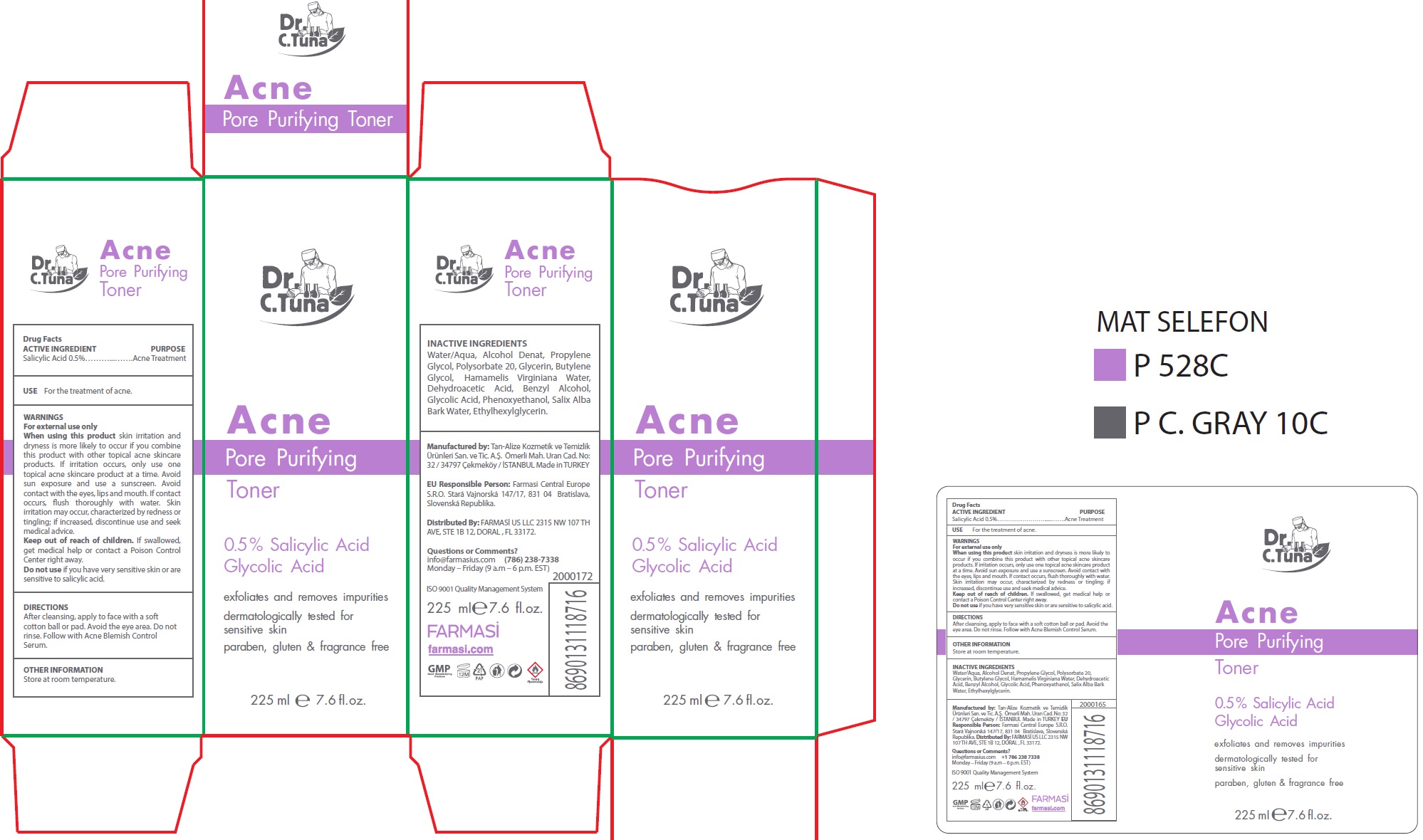
WARNINGS
For external use only
When using this product
skin irritation and dryness is more likely to occur if you combine this product with other topical acne skincare products. If irritation occurs, only use one topical acne skincare product at a time. Avoid sun exposure and use a sunscreen. Avoid contact with the eyes, lips and mouth. If contact occurs, flush thoroughly with water. Skin irritation may occur, characterized by redness or tingling; if increased, discontinue use and seek medical advice.
DIRECTIONS
After cleansing, apply to face with a soft cotton ball or pad. Avoid the eye area. Do not rinse. Follow with Acne Blemish Control Serum.