FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ExEm ® Foam is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility.
2 DOSAGE AND ADMINISTRATION
2.1 Important Pre-Administration Information
To ensure that the patient is not pregnant prior to ExEm Foam administration [see Contraindications (4) and Warnings and Precautions (5.1)]:
- Confirm that the patient has a negative pregnancy test within the 24 hours before ExEm Foam administration .
- Confirm the patient is in the pre-ovulatory phase of her menstrual cycle (cycle days 6 through 11).
2.2 Recommended Dosage
- The recommended initial dose of ExEm Foam is 2 mL to 3 mL by intrauterine infusion using a 5-Fr or larger catheter with luer connection.
- The dose may be repeated in increments of 2 mL to 3 mL, as needed, to achieve visualization of the fallopian tubes.
- Maximum total dose is 10 mL.
2.3 Preparation and Administration
The ExEm Foam kit includes the following components:
- Syringe A containing 5 mL clear Gel [polymer type A (hydroxyethyl cellulose), glycerin and purified water]
- Syringe B containing 5 mL Sterile Purified Water
- Combifix Adapter (coupling device)
Preparation
- Examine the package and do not use if package has been previously opened or damaged.
- Ensure the kit is at room temperature.
- Handle products following aseptic practices (e.g. sterile gloves).
- Generate foam by mixing Syringe A (Gel) with Syringe B (Sterile Purified Water) included in the package as described in Figure 1.
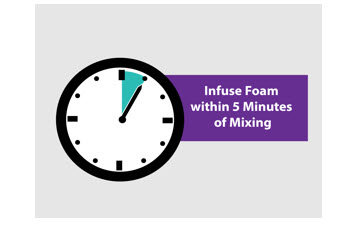
- Infuse foam within 5 minutes of reconstitution.
FIGURE 1: Reconstitution of ExEm Foam
| 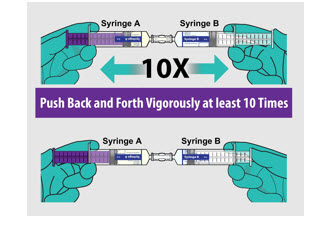 |
 |
Administration
- Administer through a 5-Fr or larger catheter with luer connection only. If there is resistance when infusing foam use a larger catheter. Do not infuse forcefully.
- Infuse 1 mL of foam to confirm proper placement of the catheter tip in the cervix and access to the uterine cavity.
- Slowly infuse 2 mL to 3 mL of the foam at a time to avoid patient discomfort.
- Discard unused portion after use.
2.4 Imaging Guidance
Conduct transvaginal ultrasound imaging in accordance with ultrasound manufacturer recommendations.
ExEm Foam in the fallopian tube will appear as an echogenic line along the length of the tube on the image.
A fallopian tube is classified as patent if ExEm Foam is observed to pass from the tube and spill into the peritoneal cavity. The tube will appear as a thin, bright line.
A fallopian tube is classified as occluded if ExEm Foam is not observed to pass from the tube and spill into the peritoneal cavity. As secondary findings, (1) there may be no bright line due to no flow into the fallopian tube, or (2) the tubal lumen may appear distended and contrast flow might be observed only in the intramural or isthmic part of the tube.
3 DOSAGE FORMS AND STRENGTHS
Intrauterine Foam, single-dose kit containing:
- Syringe A: one syringe with 5 mL clear Gel [polymer-type A (hydoxyethyl cellulose), glycerin and purified water]
- Syringe B: one syringe with 5 mL Sterile Purified Water
- One Combifix Adaptor (coupling device)
When prepared as directed ExEm Foam will contain between 10,000 to 127,000 bubbles per mL.
4 CONTRAINDICATIONS
ExEm Foam is contraindicated for use in:
- Pregnancy [see Warnings and Precautions (5.1)] .
- Patients with known or suspected lower genital tract inflammation or infection [see Warnings and Precautions (5.2)].
- Patients who have had a gynecologic procedure within the last 30 days [see Warnings and Precautions (5.2)].
- Patients with vaginal bleeding
- Due to the risk of intravasation of ExEm Foam as a result of exposure of the endometrial vessels during bleeding, and
- Due to the potential risk of endometriosis as a result of seeding the peritoneum with endometrial tissue.
- Patients with known or suspected reproductive tract neoplasia due to the risk of peritoneal spread of neoplasm .
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Fetal Harm
ExEm Foam is contraindicated for use in pregnancy due to the potential risk of fetal harm from an intrauterine procedure. To ensure that the patient is not pregnant prior to ExEm Foam administration, confirm that the patient has a negative pregnancy test within the 24 hours before ExEm Foam administration and confirm that the patient is in the pre-ovulatory phase of her menstrual cycle (cycle days 6 through 11) [see Contraindications (4)].
5.2 Risk for Post-Procedure Gynecologic Infections
There is a risk of post-procedure gynecological infections when ExEm Foam is used in sonohysterosalpingography. ExEm Foam is contraindicated for use in patients with known or suspected genital tract inflammation or infections (e.g. including pelvic inflammatory disease (PID) or suspected sexually transmitted disease) even if the patient is receiving antimicrobial therapy. ExEm Foam is contraindicated in patients who have had a gynecologic procedure within the past 30 days (e.g. curettage or conization) due to the risk of post-procedure infections [see Contraindications (4) and Adverse Reactions (6.2)] .
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug may not reflect the rates observed in practice.
The common adverse reactions associated with ExEm Foam when used as indicated in sonohysterosalpingography are: pelvic and abdominal pain; vasovagal reactions and associated symptoms such as nausea and faintness; and post-procedure spotting.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of (air polymer-type A) intrauterine foam outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gynecologic Infections: pelvic inflammatory disease, salpingitis, and tubo-ovarian abscess
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
ExEm Foam is contraindicated for use in pregnancy due to the potential risk to the fetus from an intrauterine procedure [see Contraindications (4) and Warnings and Precautions (5.1)] . There are no available data on the use of ExEm Foam in pregnant women. Animal reproduction studies have not been conducted with ExEm Foam.
8.2 Lactation
Risk Summary
There are no data on the presence of the components used to generate ExEm Foam (glycerin and hydroxyethyl cellulose) in human milk, the effects on the breastfed infant, or the effects on milk production. No adverse effects in breastfed infants are anticipated following maternal administration of ExEm Foam, based on the wide safety margin for glycerol in infants and the expected negligible absorption of hydroxyethyl cellulose [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ExEm Foam and any potential adverse effects on the breastfed infant from ExEm Foam or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Confirm that the patient has a negative pregnancy test within the 24 hours before ExEm Foam administration [see Dosage and Administration (2.1) and Use in Specific Populations (8.1)].
11 DESCRIPTION
ExEm Foam (air polymer-type A) intrauterine foam, is an ultrasound contrast agent. It is provided to the user for preparation as a single-dose kit containing:
- 5 mL sterile clear Gel [polymer-type A (80.97 mg hydroxyethyl cellulose), 434.80 mg glycerin 85%, and purified water]; with a pH of 6 to 7.5.
- 5 mL Sterile Purified Water; with a pH of 6 to 7.5.
After preparation, ExEm Foam is a milky-white, water-soluble intrauterine foam with an osmolality of approximately 462 mOsm and will contain between 10,000 to 127,000 bubbles per mL with a median size of 45.6 to 60.6 micrometers (for bubbles between 20 to 200 micrometers).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ExEm Foam (air polymer-type A) intrauterine foam is formed by mixing the clear Gel [polymer-type A (hydroxyethyl cellulose), glycerin, and purified water] with air and Sterile Purified Water, creating an echogenic contrast agent. When visualized with ultrasound, the foam appears echogenic or bright within the fallopian tubes and peritoneal cavity.
12.3 Pharmacokinetics
Assuming that a full 10 mL of ExEm Foam is used, and all glycerol is absorbed, the normal fasting endogenous glycerol plasma levels would not be affected. No literature data is found on absorption of hydroxyethyl cellulose (HEC) from the female reproductive tract and peritoneum. HEC is poorly permeable across GI epithelial mucosa; therefore, HEC is expected to exhibit very low permeability after administration and to produce negligible HEC systemic exposure.
The metabolism and excretion of HEC are not known.
13 NONCLINICAL TOXICOLOGY
No nonclinical toxicology studies have been conducted with ExEm Foam. Glycerol-hydroxyethyl cellulose (HEC) Gel is not genotoxic (Ames test) or cytotoxic (mouse lymphoma L5178Y TK+/- assay).
14 CLINICAL STUDIES
Clinical studies reported in the scientific literature have assessed the performance of sonohysterosalpingography with ExEm Foam (a technique referred to as HyFoSy) for the diagnosis of tubal patency in women with infertility. The studies used laparoscopy with chromotubation as the reference standard.
Study A compared 2D- and 3D-HyFoSy to previous laparoscopy with chromotubation in 50 women (median age 35 years) with at least 12 months of infertility (median duration of infertility 28 months). The women were randomly assigned to either HyFoSy with 2D imaging or HyFoSy with 3D imaging. The operator performing HyFoSy was blinded to the laparoscopy results. For 2D imaging, the proportion of fallopian tubes correctly identified as occluded was 80% and the proportion of tubes that were correctly identified as patent was 92%. For 3D imaging, the proportion of tubes correctly identified as occluded was 98% and the proportion of tubes that were correctly identified as patent was 91%.
Study B compared the diagnostic performance of HyFoSy with ExEm Foam using both 2D/3D-sonohysterosalpingography (2D/3D-HyFoSy) and 2D/3D-HyFoSy with high-definition flow Doppler enhancement (2D/3D-HDF-HyFoSy), to the reference standard of laparoscopy with chromotubation to establish the patency of the tubes. The study evaluated 132 women (259 fallopian tubes) with history of primary or secondary infertility who were consecutively enrolled over a period of 2 years. The mean age was 32 years and the mean duration of infertility was 2.6 years. The performance of 2D/3D-HyFoSy was similar to that of 2D/3D-HDF-HyFoSy. The performance estimates in Study B were numerically similar to those reported in Study A.
16 HOW SUPPLIED/STORAGE AND HANDLING
Supplied
ExEm Foam is supplied as a single-dose kit, NDC 73254-310-01. Each kit contains:
- Syringe A: One sterile syringe containing 5 mL of clear Gel [polymer-type A (hydroxyethyl cellulose), glycerin and purified water
- Syringe B: One sterile syringe containing 5 mL of Sterile Purified Water
- One sterile Combifix Adaptor (coupling device)
17 PATIENT COUNSELING INFORMATION
Risk for Post-Procedure Gynecologic Infections
Patients should be counseled regarding the risk of post-procedure gynecologic infections. Instruct patients to report to their healthcare provider any continued pelvic and abdominal pain, significant vaginal discharge and/or fever post-procedure [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)] .
Abdominal and Pelvic Pain
Inform patients of the potential for transient abdominal and pelvic pain during and after ExEm Foam sonohysterosalpingography [see Adverse Reactions (6.1)]
