FULL PRESCRIBING INFORMATION
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)].
EMSAM is contraindicated in patients less than 12 years of age because of an increased risk of hypertensive crisis [see Contraindications (4) and Use in Specific Populations (8.4)].
1 INDICATIONS AND USAGE
EMSAM (selegiline transdermal system) is a monoamine oxidase inhibitor (MAOI) indicated for the treatment of adults with major depressive disorder (MDD) [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Initial Treatment
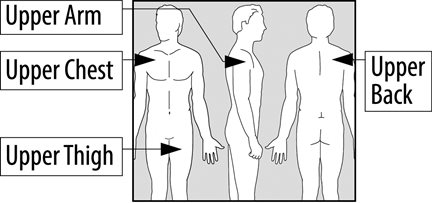
EMSAM should be applied to dry, intact skin on the upper torso (below the neck and above the waist), upper thigh or the outer surface of the upper arm once every 24 hours. The recommended starting dose and target dose for EMSAM is 6 mg per 24 hours. EMSAM has been systematically evaluated and shown to be effective in a dose range of 6 mg per 24 hours to 12 mg per 24 hours. However, the trials were not designed to assess if higher doses are more effective than the lowest effective dose of 6 mg per 24 hours. Based on clinical judgment, if dose increases are indicated for individual patients, they should occur in dose increments of 3 mg per 24 hours (up to a maximum dose of 12 mg per 24 hours) at intervals of no less than 2 weeks. Full antidepressant effect may be delayed.
Patients should be informed that tyramine-rich foods and beverages should be avoided beginning on the first day of EMSAM 9 mg per 24 hours or 12 mg per 24 hours treatment and should continue to be avoided for 2 weeks after a dose reduction to EMSAM 6 mg per 24 hours or following the discontinuation of EMSAM 9 mg per 24 hours or 12 mg per 24 hours [see Warnings and Precautions (5.3)].
2.2 Maintenance Treatment
It is generally agreed that episodes of depression require several months or longer of sustained pharmacologic therapy. Maintenance of efficacy in depressed patients on therapy with EMSAM at a dose of 6 mg per 24 hours after achieving a responder status for an average duration of about 25 days was demonstrated in a controlled trial [see Clinical Studies (14)].
The physician who elects to use EMSAM for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
2.3 Dietary Modifications Required for Patients Taking EMSAM 9 mg per 24 hours and 12 mg per 24 hours
EMSAM (selegiline transdermal system) contains a monoamine oxidase inhibitor (MAOI). MAOIs including EMSAM combined with a high tyramine diet may cause a hypertensive crisis. A hypertensive crisis can be a life-threatening condition [see Warnings and Precautions (5.3)].
The foods and beverages listed in Table 5 should be avoided beginning on the first day of EMSAM 9 mg per 24 hours or 12 mg per 24 hours treatment, and should continue to be avoided for 2 weeks after a dose reduction to EMSAM 6 mg per 24 hours or following the discontinuation of EMSAM 9 mg per 24 hours or 12 mg per 24 hours [see Drug Interactions (7.2)].
2.4 Screen for Bipolar Disorder Prior to Starting EMSAM
Prior to initiating treatment with EMSAM or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.4)].
3 DOSAGE FORMS AND STRENGTHS
EMSAM (selegiline transdermal system) is supplied as 6 mg per 24 hours (20 mg per 20 cm2), 9 mg per 24 hours (30 mg per 30 cm2) and 12 mg per 24 hours (40 mg per 40 cm2) transdermal systems (TDS).
EMSAM 6 mg per 24 hours is a translucent TDS printed with ‘EMSAM® 6mg/24h’. EMSAM 9 mg per 24 hours is a translucent TDS printed with ‘EMSAM® 9mg/24h’. EMSAM 12 mg per 24 hours is a translucent TDS printed with ‘EMSAM® 12mg/24h’.
4 CONTRAINDICATIONS
- •
- EMSAM (selegiline transdermal system) is contraindicated with selective serotonin reuptake inhibitors (SSRIs, e.g., fluoxetine, sertraline, and paroxetine); serotonin and norepinephrine reuptake inhibitors (SNRIs, e.g., venlafaxine and duloxetine); the tricyclic antidepressants clomipramine and imipramine, the opiate analgesics meperidine, tramadol, methadone, pentazocine, and propoxyphene; and the antitussive agent dextromethorphan because of a risk of serotonin syndrome when EMSAM is used with these agents [see Warnings and Precautions (5.2) and Drug Interactions (7.1)].
- •
- Carbamazepine is contraindicated with EMSAM because of a possible increased risk of hypertensive crisis [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
- •
- After stopping treatment with drugs contraindicated with EMSAM, a time period equal to 4 to 5 half-lives (approximately one week) of the drug or any active metabolite should elapse before starting therapy with EMSAM. Because of the long half-life of fluoxetine and its active metabolite, at least 5 weeks should elapse between discontinuation of fluoxetine and initiation of treatment with EMSAM.
- •
- At least 2 weeks should elapse after stopping EMSAM before starting therapy with any drug that is contraindicated with EMSAM.
- •
- EMSAM is contraindicated in patients less than 12 years of age because of the potential for a hypertensive crisis [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
- •
- EMSAM is contraindicated in patients with pheochromocytoma because MAOIs may precipitate a hypertensive crisis in such patients.
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and over 4,400 pediatric patients, the incidence of suicidal thoughts and behaviors in pediatric and young adult patients was greater in antidepressant-treated patients than in placebo-treated patients. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about antidepressant drug effect on suicide.
|
Age Range (years) |
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
|
Increases Compared to Placebo |
|
|
<18 |
14 additional patients |
|
18-24 |
5 additional patients |
|
Decreases Compared to Placebo |
|
|
25-64 |
1 fewer patient |
|
≥65 |
6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adult patients extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing EMSAM, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with concomitant use of MAOIs, such as EMSAM, with serotonergic drugs. These reactions have also been reported in patients who have discontinued serotonergic drugs and then subsequently started an MAOI [see Contraindications (4)].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular changes (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Patients should be monitored for the emergence of serotonin syndrome. Treatment with EMSAM and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive treatment should be initiated.
5.3 Blood Pressure Elevation
Tyramine-Induced Hypertensive Crisis
EMSAM inhibits the catabolism of dietary amines, such as tyramine, and has the potential to produce a hypertensive crisis following the ingestion of tyramine-rich foods or beverages [see Drug Interactions (7.2) and Clinical Pharmacology (12.2)].
Hypertensive crises, which in some cases may be fatal, are characterized by some or all of the following symptoms: occipital headache which may radiate frontally, palpitation, neck stiffness or soreness, nausea, vomiting, sweating (sometimes with fever and sometimes with cold, clammy skin), dilated pupils, and photophobia. Either tachycardia or bradycardia may be present and can be associated with constricting chest pain. Intracranial bleeding has been reported in association with the increase in blood pressure. Patients should be instructed as to the signs and symptoms of severe hypertension and advised to seek immediate medical attention if these signs or symptoms are present.
If a hypertensive crisis occurs, EMSAM should be discontinued immediately and therapy to lower blood pressure should be instituted immediately. Fever should be managed by means of external cooling. Patients must be closely monitored until symptoms have stabilized. To prevent a hypertensive crisis, patients receiving treatment with EMSAM 9 mg per 24 hours or EMSAM 12 mg per 24 hours should follow the advice regarding a low tyramine diet described in Table 5 under Dietary Modifications Required for Patients Taking EMSAM 9 mg per 24 hours and 12 mg per 24 hours [see Drug Interactions (7.2)].
Blood Pressure Elevation Related to Concomitant Medication
Carbamazepine is contraindicated with EMSAM because carbamazepine has been shown to significantly elevate selegiline levels, which may increase the risk of a hypertensive crisis [see Contraindications (4) and Drug Interactions (7.4)].
The use of EMSAM with adrenergic drugs or buspirone may produce substantial increases in blood pressure. Therefore, monitor blood pressure if EMSAM is used with any of the following drugs: buspirone, amphetamines, or cold products or weight-reducing preparations that contain sympathomimetic amines (e.g., pseudoephedrine, phenylephrine, phenylpropanolamine, and ephedrine).
5.4 Activation of Mania/Hypomania
In patients with bipolar disorder, treating a depressive episode with EMSAM or another antidepressant may precipitate a mixed/manic episode. During Phase III trials, a manic reaction occurred in 8 out of 2,036 (0.4%) patients treated with EMSAM. Prior to initiating treatment with EMSAM, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
5.5 External Heat
The effect of direct heat applied to EMSAM on the bioavailability of selegiline has not been studied. However, in theory, heat may result in an increase in the amount of selegiline absorbed from EMSAM and produce elevated serum levels of selegiline. Patients should be advised to avoid exposing the EMSAM application site to external sources of direct heat, such as heating pads or electric blankets, heat lamps, saunas, hot tubs, heated water beds, and prolonged direct sunlight.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label.
- •
- Suicidal Thoughts and Behaviors [see Warnings and Precautions (5.1)].
- •
- Serotonin Syndrome [see Contraindications (4) and Warnings and Precautions (5.2)].
- •
- Blood Pressure Elevation [see Warnings and Precautions (5.3)].
- •
- Activation of Mania/Hypomania [see Warnings and Precautions (5.4)].
- •
- External Heat [see Warnings and Precautions (5.5)].
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Patient Exposure
The premarketing development program for EMSAM included selegiline exposures in patients and/or normal subjects from two different groups of studies: 702 healthy subjects in clinical pharmacology/pharmacokinetics studies and 2,036 exposures from patients in controlled and uncontrolled major depressive disorder clinical trials. The conditions and duration of treatment with EMSAM varied and included double-blind, open-label, fixed-dose, and dose titration studies of short-term and longer-term exposures. Safety was assessed by monitoring adverse reactions, physical examinations, vital signs, body weights, laboratory analyses, and ECGs.
Adverse reactions during exposure were obtained primarily by general inquiry and recorded by clinical investigators. In the tables and tabulations that follow, standard COSTART terminology has been used to classify reported adverse reactions. The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse reaction of the type listed. A reaction was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Reactions Leading To Discontinuation of Treatment
Among 817 MDD patients treated with EMSAM at doses of either 3 mg per 24 hours (151 patients), 6 mg per 24 hours (550 patients) or 6 mg per 24 hours, 9 mg per 24 hours, and 12 mg per 24 hours (116 patients) in placebo-controlled trials of up to 8 weeks in duration, 7.1% discontinued treatment due to an adverse reaction as compared with 3.6% of 668 patients receiving placebo. The only adverse reaction associated with discontinuation, in at least 1% of EMSAM-treated patients at a rate at least twice that of placebo, was application site reaction (2% EMSAM vs. 0% placebo).
Adverse Reactions Occurring at an Incidence of 2% or More Among EMSAM-Treated Patients
Table 2 enumerates adverse reactions that occurred at an incidence of 2% or more (rounded to the nearest percent) among 817 MDD patients treated with EMSAM in doses ranging from 3 to 12 mg per 24 hours in placebo-controlled trials of up to 8 weeks in duration. Reactions included are those occurring in 2% or more of patients treated with EMSAM and for which the incidence in patients treated with EMSAM was greater than the incidence in placebo-treated patients.
One adverse reaction was associated with a reporting of at least 5% in the EMSAM group, and a rate at least twice that in the placebo group, in the pool of short-term, placebo-controlled studies: application site reactions (see Application Site Reactions, below). In one such study which utilized higher mean doses of EMSAM than that in the entire study pool, the following reactions met these criteria: application site reactions, insomnia, diarrhea, and pharyngitis.
|
||
|
Body System/Preferred Term |
EMSAM (N = 817) |
Placebo (N = 668) |
|
(% of Patients Reporting Reaction) |
||
|
Body as a Whole | ||
|
Headache |
18 |
17 |
|
Digestive | ||
|
Diarrhea |
9 |
7 |
|
Dyspepsia |
4 |
3 |
|
Nervous | ||
|
Insomnia |
12 |
7 |
|
Dry Mouth |
8 |
6 |
|
Respiratory | ||
|
Pharyngitis |
3 |
2 |
|
Sinusitis |
3 |
1 |
|
Skin | ||
|
Application Site Reaction |
24 |
12 |
|
Rash |
4 |
2 |
Application Site Reactions
In the pool of short-term, placebo-controlled major depressive disorder studies, application site reactions (ASRs) were reported in 24% of EMSAM-treated patients and 12% of placebo-treated patients. Most ASRs were mild or moderate in severity. ASRs led to dropout in 2% of EMSAM-treated patients and no placebo-treated patients. In one such study which utilized higher mean doses of EMSAM, ASRs were reported in 40% of EMSAM-treated patients and 20% of placebo-treated patients. Most of the ASRs in this study were described as erythema and most resolved spontaneously, requiring no treatment. When treatment was administered, it most commonly consisted of dermatological preparations of corticosteroids.
Sexual Dysfunction
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, in part because patients and physicians may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling are likely to underestimate their actual incidence. Table 3 shows that the incidence rates of sexual side effects in patients with major depressive disorder are comparable to the placebo rates in placebo-controlled trials.
|
Adverse Reaction |
EMSAM |
Placebo |
|
IN MALES ONLY |
||
|
(N = 304) |
(N = 256) |
|
|
Abnormal Ejaculation |
1.0% |
0.0% |
|
Decreased Libido |
0.7% |
0.0% |
|
Impotence |
0.7% |
0.4% |
|
Anorgasmia |
0.2% |
0.0% |
|
IN FEMALES ONLY |
||
|
(N = 513) |
(N = 412) |
|
|
Decreased Libido |
0.0% |
0.2% |
There are no adequately designed studies examining sexual dysfunction with EMSAM treatment.
Vital Sign Changes
EMSAM and placebo groups were compared with respect to (1) mean change from baseline in vital signs (pulse, systolic blood pressure, and diastolic blood pressure), and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. In the pool of short-term, placebo-controlled major depressive disorder studies, 3.0% of EMSAM-treated patients and 1.5% of placebo-treated patients experienced a low systolic blood pressure, defined as a reading less than or equal to 90 mmHg with a change from baseline of at least 20 mmHg. In one study which utilized higher mean doses of EMSAM, 6.2% of EMSAM-treated patients and no placebo-treated patients experienced a low standing systolic blood pressure by these criteria.
In the pool of short-term major depressive disorder trials, 9.8% of EMSAM-treated patients and 6.7% of placebo-treated patients experienced a notable orthostatic change in blood pressure, defined as a decrease of at least 10 mmHg in mean blood pressure with postural change.
Weight Changes
In placebo-controlled studies (6 to 8 weeks), the incidence of patients who experienced at least 5% weight gain or weight loss is shown in Table 4.
|
Weight Change |
EMSAM |
Placebo |
|
(N = 757) |
(N = 614) |
|
|
Gained at least 5% |
2.1% |
2.4% |
|
Lost at least 5% |
5.0% |
2.8% |
In these trials, the mean change in body weight among EMSAM-treated patients was a 1.2 lbs loss compared to 0.3 lbs gain in placebo-treated patients.
Laboratory Changes
EMSAM and placebo groups were compared with respect to (1) mean change from baseline in various serum chemistry, hematology, and urinalysis variables, and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses revealed no clinically important changes in laboratory test parameters associated with EMSAM.
Electrocardiogram Changes
Electrocardiograms (ECGs) from EMSAM (N = 817) and placebo (N = 668) groups in controlled studies were compared with respect to (1) mean change from baseline in various ECG parameters, and (2) the incidence of patients meeting criteria for clinically significant changes from baseline in these variables.
No clinically meaningful changes in ECG parameters from baseline to final visit were observed for patients in controlled studies.
Other Reactions Observed During the Premarketing Evaluation of EMSAM
The following listing does not include reactions: 1) already listed elsewhere in labeling, 2) for which a causal relationship to drug was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
Cardiovascular System: Tachycardia.
Digestive System: Anorexia.
Nervous System: Agitation, amnesia, tremor, twitching.
Skin and Appendages: Pruritus.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of EMSAM.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System: Convulsion and hypoesthesia.
Psychiatric System: Disorientation, hallucination (visual), and tension.
7 DRUG INTERACTIONS
7.1 Serotonergic Drugs
Serious, sometimes fatal, central nervous system (CNS) toxicity referred to as the “serotonin syndrome” has been reported with the combination of nonselective MAOIs and serotonergic drugs. Use of EMSAM with these drugs is contraindicated [see Contraindications (4) and Warnings and Precautions (5.2)].
7.2 Tyramine
EMSAM has the capacity to inhibit intestinal MAO, which is responsible for the catabolism of tyramine in food and beverages. As a result of this inhibition, large amounts of tyramine may enter the systemic circulation and precipitate a sudden, large rise in blood pressure or hypertensive crisis [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.2)].
A diet low in tyramine content may be necessary to avoid this interaction. Studies to evaluate the potential for EMSAM to inhibit tyramine metabolism have been conducted and, overall, the data for EMSAM 6 mg per 24 hours support a recommendation that a modified diet is not required at this dose. Due to the more limited data available for EMSAM 9 mg per 24 hours and the results from the Phase I tyramine challenge study in fed volunteers administered EMSAM 12 mg per 24 hours, patients receiving these doses should follow Dietary Modifications Required for Patients Taking EMSAM 9 mg per 24 hours and 12 mg per 24 hours below [see Clinical Pharmacology (12.2)].
Dietary Modifications Required for Patients Taking EMSAM 9 mg per 24 hours and 12 mg per 24 hours
The foods and beverages listed in Table 5 should be avoided beginning on the first day of EMSAM 9 mg per 24 hours or 12 mg per 24 hours treatment, and should continue to be avoided for 2 weeks after a dose reduction to EMSAM 6 mg per 24 hours or following the discontinuation of EMSAM 9 mg per 24 hours or 12 mg per 24 hours.
|
||
|
Class of Food and Beverage |
Tyramine-Rich Foods and Beverages to Avoid |
Acceptable Foods and Drinks, Containing No or Little Tyramine |
|
Meat, Poultry, and Fish |
Air dried, aged and fermented meats, sausages and salamis (including cacciatore, hard salami and mortadella); pickled herring; and any spoiled or improperly stored meat, poultry, and fish (e.g., foods that have undergone changes in coloration, odor, or become moldy); spoiled or improperly stored animal livers |
Fresh meat, poultry, and fish, including fresh processed meats (e.g., lunch meats, hot dogs, breakfast sausage, |
|
Vegetables |
Broad bean pods (fava bean pods) |
All other vegetables |
|
Dairy |
Aged cheeses |
Processed cheeses, mozzarella, ricotta cheese, cottage cheese, and yogurt |
|
Beverages |
All varieties of tap beer and beers that have not been pasteurized so as to allow for ongoing fermentation |
Concomitant use of alcohol with EMSAM is not recommended. (Bottled and canned beers and wines contain little or no tyramine.) |
|
Miscellaneous |
Concentrated yeast extract (e.g., Marmite), sauerkraut, most soybean products (including soy sauce and tofu), OTC supplements containing tyramine |
Brewer’s yeast, baker’s yeast, soy milk, commercial chain restaurant pizzas prepared with cheeses low in tyramine |
7.3 Sympathomimetic Amines and Buspirone
The use of EMSAM with sympathomimetic amines or buspirone may produce substantial elevations in blood pressure. Therefore, monitor blood pressure if EMSAM is used with any of the following drugs: buspirone, amphetamines, and cold products or weight-reducing preparations that contain sympathomimetic amines (e.g., pseudoephedrine, phenylephrine, phenylpropanolamine, and ephedrine).
7.4 Effect of Other Drugs on EMSAM
Carbamazepine is contraindicated with MAOIs, including selegiline [see Contraindications (4), Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
No dose adjustment for EMSAM is needed when EMSAM is used concomitantly with alcohol, alprazolam, ibuprofen, olanzapine, risperidone, levothyroxine, and CYP3A4 inhibitors (e.g., ketoconazole). No clinically meaningful change in selegiline exposure was seen when EMSAM was co-administered with alcohol, alprazolam, ibuprofen, olanzapine, risperidone, levothyroxine, and ketoconazole [see Clinical Pharmacology (12.3)].
7.5 Effect of EMSAM on Other Drugs
Use of alcohol while taking EMSAM is not recommended, even though EMSAM has not been shown to increase the impairment of mental and motor skills caused by alcohol (0.75 mg per kg) [see Clinical Pharmacology (12.3)].
Monitor blood pressure if sympathomimetic agents (e.g., phenylpropanolamine (PPA) or pseudoephedrine) are used with EMSAM, even though selegiline does not appear to affect the pharmacokinetics of PPA or pseudoephedrine [see Clinical Pharmacology (12.3)].
No dose adjustment of alprazolam, ibuprofen, levothyroxine, olanzapine, risperidone, warfarin, or strong CYP3A4 inhibitors (e.g., ketoconazole) is necessary when these drugs are used in combination with EMSAM. EMSAM had no clinically relevant effect on pharmacokinetics of these drugs.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data on EMSAM use in pregnant women are not sufficient to inform a drug-associated risk of adverse pregnancy-related outcomes. In animal embryo-fetal development studies, transdermal administration of selegiline to rats and rabbits at doses up to 60 and 64 times the maximum recommended human dose (MRHD) respectively, produced slight increases in malformations in both rats and rabbits, and decreased fetal weight, delayed ossification, and embryo-fetal post-implantation loss in rats. Most of these effects were seen at the high dose in both rats and rabbits. These effects were not seen at 8 times and 16 times the MRHD in rats and rabbits, respectively. In a pre-natal and post-natal development study, transdermal administration of selegiline in rats at doses 8, 24, and 60 times MRHD produced a decrease in pup weight and survival at the medium and high doses, an increase in the number of stillborn pups at the high dose, and delayed neurobehavioral and sexual development in pups
at all doses. A persistent effect on reproductive performance of pups born to mothers treated at the high dose was evident (see Data). When treating a pregnant woman with EMSAM, the physician should carefully consider both the potential risks of taking an MAOI, particularly the risk of hypertensive crisis during pregnancy, along with the established benefits of treating depression with an antidepressant.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
A prospective longitudinal study was conducted of 201 pregnant women with a history of major depression, who were either on antidepressants or had received antidepressants less than 12 weeks prior to their last menstrual period, and were in remission. Women who discontinued antidepressant medication during pregnancy showed a significant increase in relapse of their major depression compared to those women who remained on antidepressant medication throughout pregnancy.
Data
Animal Data
In an embryofetal development study, rats were treated with transdermal selegiline during the period of organogenesis at doses of 10, 30, and 75 mg/kg/day (8, 24, and 60 times the MRHD of EMSAM [12 mg/24 hours] on a mg/m2 basis). At the highest dose there was a decrease in fetal weight and slight increases in malformations, delayed ossification (also seen at the mid dose), and embryofetal post-implantation loss. Concentrations of selegiline and its metabolites in fetal plasma were generally similar to those in maternal plasma.
In an embryofetal development study, rabbits were treated with transdermal selegiline during the period of organogenesis at doses of 2.5, 10, and 40 mg/kg/day (4, 16, and 64 times the MRHD on a mg/m2 basis). A slight increase in visceral malformations was seen at the high dose.
In a prenatal and postnatal development study, rats were treated with transdermal selegiline at doses of 10, 30, and 75 mg/kg/day (8, 24, and 60 times the MRHD on a mg/m2 basis) on days 6 to 21 of gestation and days 1 to 21 of the lactation period. An increase in post-implantation loss was seen at the mid and high doses, and an increase in stillborn pups was seen at the high dose. Decreases in pup weight (throughout lactation and postweaning periods) and survival (throughout lactation period), delayed pup physical development, and pup epididymal and testicular hypoplasia, were seen at the mid and high doses. Delayed neurobehavioral and sexual development was seen at all doses. Adverse effects on pup reproductive performance, as evidenced by decreases in implantations and litter size, were seen at the high dose. These findings suggest persistent effects on the offspring of treated dams. A no-effect dose was not established in this study for developmental toxicity.
8.2 Lactation
Risk Summary
There is no information regarding the presence of selegiline in human milk, or on its effects on milk production or the breastfed infant. Selegiline and its metabolites are present in the milk of lactating rats (see Data).
Because of the potential for serious adverse reactions in breastfed infants from EMSAM, including the potential for hypertensive crisis, advise a woman that breastfeeding is not recommended during treatment with EMSAM and for 5 days after the final dose.
Data
In a prenatal and postnatal development study where rats were treated with transdermal selegiline at doses approximately 8, 24, and 60 times the MRHD on days 6 to 21 of gestation and days 1 to 21 of the lactation period, concentrations of selegiline and its metabolites in milk were approximately 15 and 5 times, respectively, the concentrations in maternal plasma.
8.4 Pediatric Use
Use of EMSAM in patients less than 12 years of age is contraindicated because of the potential for a hypertensive crisis [see Contraindications (4)].
Limited pharmacokinetic data with doses lower than in the commercially available formulations suggest that children under age 12 may be exposed to increased levels of selegiline compared to adolescents and adults, administered with and without dietary modifications, therefore, there may be an increased risk of hypertensive crisis, even at the lowest dose of EMSAM.
Efficacy has not been established in pediatric patients ages 12 to 17 years with MDD and EMSAM is not recommended for use in this age range [see Clinical Pharmacology (12.3)].
A multi-center, randomized, double-blind, placebo-controlled, flexible-dose trial in 308 adolescents (ages 12 to 17 years) with MDD failed to demonstrate the efficacy of EMSAM. Diagnosis of major depressive disorder (single episode or recurrent, moderate to severe) was based on according DSM-IV criteria and Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS). Enrolled patients had a Children’s Depression Rating Scale-Revised of ≥ 45 at the screening visit. Trial participants were randomized 1:1 to either EMSAM or matching placebo without forced titration for a period of 12 weeks. Active treatment consisted of EMSAM transdermal system at a dose of 6 mg per 24 hours, 9 mg per 24 hours, or 12 mg per 24 hours. The primary efficacy endpoint was the difference in total score on the Children’s Depression Rating Scale-Revised (CDRS-R) from baseline to the end of study (EOS) (Week 12). There was no observed difference in effect on CDRS-R Total Score at Week 12 (EOS) between treatments. The mean reduction in CDRS-R Total Score was 21.4 in the EMSAM-treated subjects and 21.5 in those receiving placebo treatment. Safety endpoints included physical examination, 12-lead electrocardiogram, respiration rate, temperature, supine and standing blood pressure and heart rate, application site assessments, and adverse events. Overall, safety findings were similar to those observed in EMSAM trials conducted in adults. Treatment-emergent adverse events reported by at least 5% of EMSAM-treated patients at a rate at least twice the placebo rate were insomnia (6%, 3%) and upper respiratory tract infection (7%, 3%).
8.5 Geriatric Use
The recommended dose of EMSAM for elderly patients (65 years and older) is 6 mg per 24 hours daily. The effect of age on the pharmacokinetics or metabolism of selegiline after administration of EMSAM has not been systematically evaluated. One hundred ninety-eight (198) elderly (65 years of age and older) patients participated in clinical studies with EMSAM 6 mg per 24 hours to 12 mg per 24 hours. There were no overall differences in effectiveness between elderly and younger patients. In short-term, placebo-controlled depression trials, patients age 50 and older appeared to be at higher risk for rash (4.4% EMSAM vs. 0% placebo) than younger patients (3.4% EMSAM vs. 2.4% placebo).
8.6 Gender
No adjustment of EMSAM dosage based on gender is needed. No gender differences have been observed in the pharmacokinetics or metabolism of selegiline during administration of EMSAM.
8.7 Reduced Hepatic Function
No adjustment of EMSAM dosage is required in patients with mild liver impairment (Child-Pugh 5-6 points) or moderate liver impairment (Child-Pugh 7-9 points). After a single administration of EMSAM 6 mg per 24 hours in eight patients with mild or moderate liver impairment, no differences in either the metabolism or pharmacokinetic behavior of selegiline or its metabolites were observed as compared with data of normal subjects. EMSAM has not been studied in patients with severe liver impairment (Child-Pugh 10-15 points).
8.8 Reduced Renal Function
No adjustment of EMSAM dosage is required in patients with mild renal impairment (eGFR 60-89 mL/min/1.73 m2), moderate renal impairment (eGFR 30-59 mL/min/1.73 m2), or severe renal impairment (eGFR 15-29 mL/min/1.73 m2). Data from a single dose study examining the pharmacokinetics of EMSAM 6 mg per 24 hours in 12 patients with renal impairment suggest that mild, moderate, or severe renal impairment does not affect the pharmacokinetics of selegiline after transdermal application. EMSAM has not been studied in patients with end-stage renal disease (eGFR < 15 mL/min/1.73 m2 or requiring dialysis).
10 OVERDOSAGE
10.1 Signs and Symptoms
EMSAM overdosage may resemble overdosage with other nonselective, oral MAOI antidepressants and present with any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, coma, rapid and irregular pulse, hypertension, hypotension and vascular collapse, precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
10.2 Management of Overdose
There are no specific antidotes for EMSAM.
If symptoms of overdosage occur, immediately remove the EMSAM system and institute appropriate supportive therapy. For contemporary information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222.
Delays of up to 12 hours between ingestion of drug and the appearance of signs may occur, and peak effects may not be observed for 24 to 48 hours. Since death has been reported following overdosage with MAOI agents, hospitalization with close monitoring during this period is strongly recommended.
In order to avoid the occurrence of hypertensive crisis (“cheese reaction”), dietary tyramine should be restricted for several weeks beyond recovery to permit regeneration of the peripheral MAO-A isoenzyme.
11 DESCRIPTION
EMSAM® contains selegiline, a MAOI antidepressant. When applied to intact skin, EMSAM is designed to transdermally deliver selegiline over a 24-hour period.
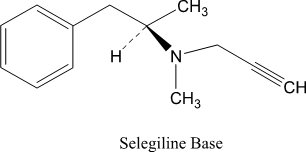
Selegiline base is a colorless to yellow liquid, chemically described as (-)-(N)-Methyl-N-[(1R)-1-methyl-2-phenylethyl]prop-2-yn-1-amine. It has a molecular formula of C13H17N and a molecular weight of 187.30. The structural formula is:
EMSAM transdermal systems are available in three strengths that deliver approximately 6 mg, 9 mg, or 12 mg of selegiline over 24 hours. Each corresponding system has an active surface area of 20 cm2, 30 cm2, or 40 cm2 containing 20, 30, or 40 mg of selegiline, respectively. The composition of the systems per unit area is identical.
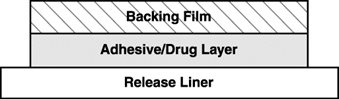
EMSAM is a matrix-type transdermal system composed of three layers as illustrated in Figure 1 below. Layer 1 is the Backing Film that provides occlusivity, physical integrity and protects the adhesive/drug layer. Layer 2 is the Adhesive/Drug Layer. Layer 3 consists of side-by-side release liners that are peeled off and discarded by the patient prior to applying EMSAM. The inactive ingredients are acrylic adhesive, ethylene vinyl acetate/polyethylene, polyester, polyurethane, and silicone coated polyester.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of selegiline (the drug substance of EMSAM) as an antidepressant is not fully understood, but is presumed to be linked to potentiation of monoamine neurotransmitter activity in the central nervous system (CNS) resulting from its irreversible inhibition of the enzyme monoamine oxidase (MAO).
12.2 Pharmacodynamics
MAO exists as two isoenzymes, referred to as MAO-A and MAO-B. Selegiline has a greater affinity for MAO-B, compared to MAO-A. However, at antidepressant doses, selegiline inhibits both isoenzymes. In an in vivo animal model used to test for antidepressant activity (Forced Swim Test), selegiline administered by transdermal system exhibited antidepressant properties only at doses that inhibited both MAO-A and MAO-B activity in the brain. In the CNS, MAO-A and MAO-B play important roles in the catabolism of neurotransmitter amines such as norepinephrine, dopamine, and serotonin, as well as neuromodulators such as phenylethylamine.
Receptor Binding
In in vitro receptor binding assays, selegiline has demonstrated affinity for the human recombinant adrenergic α2B receptor (Ki = 0.3 mcM). No affinity [Ki greater than 10 mcM] was noted at dopamine receptors, adrenergic β3, glutamate, muscarinic M1-M5, nicotinic, or rolipram receptor/sites.
Interaction with Tyramine
Selegiline (the drug substance of EMSAM) is an irreversible inhibitor of monoamine oxidase (MAO), a ubiquitous intracellular enzyme. MAO exists as two isoenzymes, referred to as MAO-A and MAO-B. Selegiline shows greater affinity for MAO-B; however, as selegiline concentration increases, this selectivity is lost with resulting dose-related inhibition of MAO-A. Intestinal MAO is predominantly type A, while in the brain both isoenzymes exist.
MAO plays a vital physiological role in terminating the biological activity of both endogenous and exogenous amines. In addition to their role in the catabolism of monoamines in the CNS, MAOs are also important in the catabolism of exogenous amines found in a variety of foods and drugs. MAO in the gastrointestinal tract (primarily type A) provides protection from exogenous amines with vasopressor actions, such as tyramine, which if absorbed intact can cause a hypertensive crisis, the so-called “cheese reaction”. If a large amount of tyramine is absorbed systemically, it is taken up by adrenergic neurons and causes norepinephrine release from neuronal storage sites with resultant elevation of blood pressure. While most foods contain negligible amounts or no tyramine, certain food products may contain large amounts of tyramine that represent a potential risk for hypertensive crisis [see Warnings and Precautions (5.3)].
To define the risk of hypertensive crises with use of EMSAM, several Phase I tyramine challenge studies were conducted both with and without food. Fourteen tyramine challenge studies including 214 healthy subjects (age range 18 to 65; 31 subjects greater than 50 years of age) were conducted to determine the pressor effects of oral tyramine with concurrent EMSAM treatment (6 mg per 24 hours to 12 mg per 24 hours), measured as the dose of tyramine required to raise systolic blood pressure by 30 mmHg (TYR30). Studies were conducted with and without concomitant administration of food. Studies conducted with food are most relevant to clinical practice since tyramine typically will be consumed in food. A high-tyramine meal is considered to contain up to 40 mg of tyramine.
One study using a crossover design in 13 subjects investigated tyramine pressor doses (TYR30) after administration of EMSAM 6 mg per 24 hours and oral selegiline (5 mg twice daily) for 9 days. Mean pressor doses (TYR30) of tyramine capsules administered without food were 338 mg and 385 mg in subjects treated with EMSAM and oral selegiline, respectively.
Another study using a crossover design in 10 subjects investigated tyramine pressor doses after administration of EMSAM 6 mg per 24 hours or tranylcypromine 30 mg per day for 10 days. Mean pressor doses (TYR30) of tyramine capsules administered without food were 270 mg in subjects treated with EMSAM 6 mg per 24 hours and 10 mg in subjects treated with tranylcypromine.
In a third crossover study, tyramine without food was administered to 12 subjects. The mean tyramine pressor doses (TYR30) after administration of EMSAM 6 mg per 24 hours for 9 and 33 days were 292 mg and 204 mg, respectively. The lowest pressor dose was 50 mg in one subject in the 33-day group.
Tyramine pressor doses were also studied in 11 subjects after extended treatment with EMSAM 12 mg per 24 hours. At 30, 60, and 90 days, the mean pressor doses (TYR30) of tyramine administered without food were 95 mg, 72 mg, and 88 mg, respectively. The lowest pressor dose without food was 25 mg in three subjects at day 30 while on EMSAM 12 mg per 24 hours. Eight subjects from this study, with a mean tyramine pressor dose of 64 mg at 90 days, were subsequently administered tyramine with food, resulting in a mean pressor dose of 172 mg (2.7 times the mean pressor dose observed without food, p less than 0.003).
With the exception of one study (N = 153), the Phase III clinical development program was conducted without requiring a modified diet (N = 2,553, 1,606 at 6 mg per 24 hours, and 947 at 9 mg per 24 hours or 12 mg per 24 hours). No hypertensive crises were reported in any patient receiving EMSAM.
Overall, the data for EMSAM 6 mg per 24 hours support a recommendation that a modified diet is not required at this dose. Due to the more limited data available for EMSAM 9 mg per 24 hours and the results from the Phase I tyramine challenge study in fed volunteers administered EMSAM 12 mg per 24 hours, patients receiving these doses should follow Dietary Modifications Required for Patients Taking EMSAM 9 mg per 24 hours and 12 mg per 24 hours [see Warnings and Precautions (5.3)].
12.3 Pharmacokinetics
Absorption
Following dermal application of EMSAM to humans, 25% to 30% of the selegiline content on average is delivered systemically over 24 hours (range approximately 10% to 40%). Consequently, the degree of drug absorption may be 1/3 higher than the average amounts of 6 mg to 12 mg per 24 hours. Transdermal dosing results in significantly higher exposure to selegiline with significantly lower exposure for all metabolites when compared to oral dosing, due to extensive first-pass metabolism. In a 10-day study with daily administration of EMSAM to healthy male and female volunteers, steady-state selegiline plasma concentrations indicated selegiline concentration-time profiles were comparable when EMSAM is applied to the upper torso or upper thigh, and absorption from these two sites of administration was equivalent.
Distribution
Following dermal application of radiolabeled selegiline to laboratory animals, selegiline is rapidly distributed to all body tissues. Selegiline rapidly penetrates the blood-brain barrier.
In humans, selegiline is approximately 90% bound to plasma protein over a 2 to 500 ng per mL concentration range. Selegiline does not accumulate in the skin.
In vivo Metabolism
Transdermally absorbed selegiline (via EMSAM) is not metabolized in human skin and does not undergo extensive first-pass metabolism. Selegiline is extensively metabolized by several CYP450-dependent enzyme systems (see In vitro Metabolism). Selegiline is metabolized initially via N-dealkylation or N-depropargylation to form N-desmethylselegiline or R(-)-methamphetamine, respectively. Both of these metabolites can be further metabolized to R(-)-amphetamine. These metabolites are all levorotatory (l-)enantiomers and no racemic biotransformation to the dextrorotatory form (i.e., S(+)-amphetamine or S(+)-methamphetamine) occurs. R(-)-methamphetamine and R(-)-amphetamine are mainly excreted unchanged in urine.
In vitro Metabolism
In vitro studies utilizing human liver microsomes demonstrated that several CYP450-dependent enzymes are involved in the metabolism of selegiline and its metabolites. CYP2B6, CYP2C9, CYP3A4 and CYP3A5 appeared to be the major contributing enzymes in the formation of R(-)-methamphetamine from selegiline, with CYP2A6 having a minor role. CYP2A6, CYP2B6, CYP3A4 and CYP3A5 appeared to contribute to the formation of R(-)-amphetamine from N-desmethylselegiline.
The potential for selegiline or N-desmethylselegiline to inhibit individual CYP450-dependent enzyme pathways was also examined in vitro with human liver microsomes. Each substrate was examined over a concentration range of 2.5 to 250 mcM. Consistent with competitive inhibition, both selegiline and N-desmethylselegiline caused a concentration dependent inhibition of CYP2D6 at 10 to 250 mcM and CYP3A4 and CYP3A5 at 25 to 250 mcM. CYP2C19 and CYP2B6 were also inhibited at concentrations of 100 mcM or greater. All inhibitory effects of selegiline and N-desmethylselegiline occurred at concentrations that are several orders of magnitude higher than concentrations seen clinically (highest predose concentration observed at a dose of 12 mg per 24 hours at steady-state was 0.046 mcM) [see Drug Interactions (7)].
Excretion
Approximately 10% and 2% of a radiolabeled dose applied dermally, as a DMSO solution, was recovered in urine and feces respectively, with at least 63% of the dose remaining unabsorbed. The remaining 25% of the dose was unaccounted for. Urinary excretion of unchanged selegiline accounted for 0.1% of the applied dose with the remainder of the dose recovered in urine being metabolites.
The systemic clearance of selegiline after intravenous administration was 1.4 L per min, and the mean half-lives of selegiline and its three metabolites, R(-)-N-desmethylselegiline, R(-)-amphetamine, and R(-)-methamphetamine, ranged from 18 to 25 hours.
Population Subgroups
Age
EMSAM should not be used in patients less than 18 years of age [see Use in Specific Populations (8.4)].
Stratification of exposure data following treatment with EMSAM indicated that pre-dose (trough) selegiline plasma concentrations at steady state appeared higher (p = 0.12) in children aged < 12 years old, compared to adolescents aged ≥ 12 years as shown in Table 6.
|
Trough Concentration, pg/mL |
||
|
6 to 11 year old age group |
12 to 14 year old age group |
|
|
(N = 6) |
(N = 4) |
|
|
Mean (SD) |
2,562 (974) |
1,821 (146) |
Drug-Drug Interactions
Alcohol
The pharmacokinetics and pharmacodynamics of alcohol (0.75 mg per kg) alone or in combination with EMSAM 6 mg per 24 hours for 7 days of treatment was examined in 16 healthy volunteers. No clinically significant differences were observed in the pharmacokinetics or pharmacodynamics of alcohol or the pharmacokinetics of selegiline during co-administration. Although EMSAM has not been shown to increase the impairment of mental and motor skills caused by alcohol (0.75 mg per kg) and failed to alter the pharmacokinetic properties of alcohol, patients should be advised that the use of alcohol is not recommended while taking EMSAM [see Drug Interactions (7.4) and (7.5)].
Alprazolam
In subjects who had received EMSAM 6 mg per 24 hours for 7 days, co-administration with alprazolam (15 mg per day), a CYP3A4 and CYP3A5 substrate, did not affect the pharmacokinetics of alprazolam or selegiline [see Drug Interactions (7.4) and (7.5)].
Carbamazepine
Carbamazepine is an enzyme inducer and typically causes decreases in drug exposure; however, approximately 2-fold increased systemic exposure of selegiline and its metabolites, L-amphetamine and L-methamphetamine were seen after single application of EMSAM 6 mg per 24 hours in subjects who had received carbamazepine (400 mg per day) for 14 days. Changes in plasma selegiline concentrations were nearly 2-fold and variable across the subject population. Such increases may increase the risk of a hypertensive crisis when carbamazepine is used with EMSAM at any dose [see Contraindications (4), Warnings and Precautions (5.3) and Drug Interaction (7.4)].
Ibuprofen
In subjects who had received EMSAM 6 mg per 24 hours for 11 days, combined administration with the CYP2C9 substrate ibuprofen (800 mg single dose) did not affect the pharmacokinetics of either selegiline or ibuprofen [see Drug Interactions (7.4) and (7.5)].
Ketoconazole
Seven-day treatment with ketoconazole (200 mg per day), a potent inhibitor of CYP3A4, did not affect the steady-state pharmacokinetics of selegiline in subjects who received EMSAM 6 mg per 24 hours for 7 days and no differences in the pharmacokinetics of ketoconazole were observed [see Drug Interactions (7.4) and (7.5)].
Levothyroxine
In healthy subjects who had received EMSAM 6 mg per 24 hours for 10 days, single dose administration with levothyroxine (150 mcg) did not alter the pharmacokinetics of either selegiline or levothyroxine [see Drug Interactions (7.4) and (7.5)].
Olanzapine
In subjects who had received EMSAM 6 mg per 24 hours for 10 days, co-administration with olanzapine, a substrate for CYP1A2, CYP2D6, and possibly CYP2A6, did not affect the pharmacokinetics of selegiline or olanzapine [see Drug Interactions (7.4) and (7.5)].
Phenylpropanolamine (PPA)
In subjects who had received EMSAM 6 mg per 24 hours for 9 days, co-administration with PPA (25 mg every 4 hours for 24 hours) did not affect the pharmacokinetics of PPA. There was a higher incidence of significant blood pressure elevations with the co-administration of EMSAM and PPA than with PPA alone, suggesting a possible pharmacodynamic interaction [see Drug Interactions (7.4) and (7.5)].
Pseudoephedrine
EMSAM 6 mg per 24 hours for 10 days, co-administered with pseudoephedrine (60 mg, 3 times a day) did not affect the pharmacokinetics of pseudoephedrine. There were no clinically significant changes in blood pressure during pseudoephedrine administration alone, or in combination with EMSAM [see Drug Interactions (7.4) and (7.5)].
Risperidone
In subjects who had received EMSAM 6 mg per 24 hours for 10 days, co-administration with risperidone (2 mg per day for 7 days), a substrate for CYP2D6, did not affect the pharmacokinetics of selegiline or risperidone [see Drug Interactions (7.4) and (7.5)].
Warfarin
Warfarin is a substrate for CYP2C9 and CYP3A4 metabolism pathways. In healthy volunteers titrated with Coumadin® (warfarin sodium) to clinical levels of anticoagulation (INR of 1.5 to 2), co-administration with EMSAM 6 mg per 24 hours for 7 days did not affect the pharmacokinetics of the individual warfarin enantiomers. EMSAM did not alter the clinical pharmacodynamic effects of warfarin as measured by INR, Factor VII or Factor X levels [see Drug Interactions (7.4) and (7.5)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a dermal carcinogenicity study in CD-1 mice, selegiline (the drug substance of EMSAM) was administered daily for 2 years at the same skin site at dose levels of 20, 70, and 200 mg per kg per day (dissolved in acetone). The incidence of systemic tumors was not increased and the high dose provided systemic exposures to selegiline and its three metabolites in mice that were greater than 40 times the exposures in humans at the maximum recommended human dose (MRHD). The incidence of squamous cell carcinoma was slightly increased on treated skin of mice administered the high dose. This finding was associated with an increased incidence of epithelial hyperplasia, dyskeratosis/hyperkeratosis and inflammation.
In an oral carcinogenicity study in rats, selegiline given in the diet for 104 weeks was not carcinogenic up to the highest evaluable dose tested (3.5 mg per kg per day), which exposed rats to systemic levels of selegiline and its three metabolites that were comparable to those in humans at the MRHD.
Mutagenesis
Selegiline induced mutations and chromosomal damage when tested in the in vitro mouse lymphoma assay with and without metabolic activation. Selegiline was negative in the Ames assay, the in vitro mammalian chromosome aberration assay in human lymphocytes, and the in vivo oral mouse micronucleus assay.
Impairment of Fertility
A mating and fertility study was conducted in male and female rats at transdermal doses of 10, 30, and 75 mg per kg per day of selegiline (8, 24, and 60 times the maximum recommended human dose of EMSAM [12 mg per 24 hours] on a mg per m2 basis). Slight decreases in sperm concentration and total sperm count were observed at the high dose; however, no significant adverse effects on fertility or reproductive performance were observed.
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
The efficacy of EMSAM as a treatment for major depressive disorder was established in two placebo-controlled studies of 6 and 8 weeks duration in adult outpatients (ages 18 to 70 years) meeting DSM-IV criteria for major depressive disorder. In both studies, patients were randomized to double-blind treatment with EMSAM or placebo. The 6-week trial (N = 176) showed that EMSAM 6 mg per 24 hours was statistically significantly more effective than placebo on the 17-item Hamilton Depression Rating Scale (HAM-D) total score (Study 1 in Table 7). In an 8-week dose titration trial, depressed patients (N = 265), who received EMSAM or placebo at a starting dose of 6 mg per 24 hours, with possible increases to 9 mg per 24 hours or 12 mg per 24 hours based on clinical response, showed significant improvement compared with placebo on the primary outcome measure, the 28-item HAM-D total score (Study 2 in Table 7).
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. | |||||
|
|||||
|
Study Number
|
Treatment Group |
Number of Patients |
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Difference* (95% CI) |
|
Study 1 |
EMSAM (6 mg) |
89 |
22.9 (2.1) |
-9.0 (0.8) |
-2.5 |
|
[HAMD-17] |
Placebo |
88 |
23.3 (2.9) |
-6.5 (0.8) |
(-4.6, -0.4) |
|
Study 2 |
EMSAM |
132 |
28.3 (3.7) |
-10.9 (0.8) |
-2.4 |
|
[HAMD-28] |
Placebo |
133 |
28.5 (3.9) |
-8.6 (0.8) |
(-4.5, -0.3) |
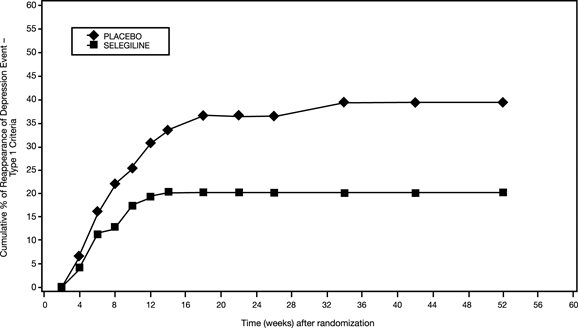
In another trial (Study 3), 322 patients meeting DSM-IV criteria for major depressive disorder who had responded during an initial 10-week open-label treatment phase for about 25 days, on average, to EMSAM 6 mg per 24 hours were randomized either to continuation of EMSAM at the same dose (N = 159) or to placebo (N = 163) under double-blind conditions for observation of relapse. About 52% of the EMSAM-treated patients, as well as about 52% of the placebo-treated patients, had discontinued treatment by week 12 of the double-blind phase. Response during the open-label phase was defined as 17-item HAM-D total score less than 10 at either week 8 or 9 and at week 10 of the open-label phase. Relapse during the double-blind phase was defined as follows: (1) a 17-item HAM-D score of 14 or greater, (2) a CGI-S score of 3 or greater (with at least a 2-point increase from double-blind baseline), and (3) meeting DSM-IV criteria for major depressive disorder on two consecutive visits at least 11 days apart. In the double-blind phase, patients receiving continued EMSAM experienced a significantly longer time to relapse (Figure 2).
Figure 2. Kaplan-Meier Estimates of Cumulative Percent of Patients with Relapse (Study 3)
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender, or race.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
EMSAM (selegiline transdermal system) is a transdermal system with the following strengths, sizes, color, backing film printing and presentation:
|
Features |
Strengths |
||
|
6 mg per 24 hours |
9 mg per 24 hours |
12 mg per 24 hours |
|
|
EMSAM Size |
20 mg per 20 cm2 |
30 mg per 30 cm2 |
40 mg per 40 cm2 |
|
Color |
Translucent |
Translucent |
Translucent |
|
Backing Film |
EMSAM® 6mg/24h |
EMSAM® 9mg/24h |
EMSAM® 12mg/24h |
|
NDC number |
NDC 49502-900-30 |
NDC 49502-901-30 |
NDC 49502-902-30 |
|
Presentation |
Box of 30 transdermal systems |
Box of 30 transdermal systems |
Box of 30 transdermal systems |
Storage and Handling
Store at 20° to 25° C (68° to 77° F). [See USP Controlled Room Temperature.] Do not store outside of the sealed pouch.
Apply immediately upon removal from the protective pouch. Discard used EMSAM in household trash in a manner that prevents accidental application or ingestion by children, pets or others.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide and Instructions for Use).
Advise patients and their caregivers about the benefits and risks associated with treatment with EMSAM and counsel them in its appropriate use. Advise patients and their caregivers to read the Medication Guide and assist them in understanding its contents. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking EMSAM.
Suicide Risk: Advise patients and caregivers to look for the emergence of suicidal ideation and behavior, especially early during treatment and when the dose is adjusted up or down [see Boxed Warning and Warnings and Precautions (5.1)].
Tyramine Reactions: Patients should be advised that tyramine-rich foods and beverages should be avoided while on EMSAM 9 mg per 24 hours or EMSAM 12 mg per 24 hours, and for 2 weeks following discontinuation of EMSAM at these doses because of the risk of a tyramine reaction [see Warnings and Precautions (5.3), Drug Interactions (7.2), and Clinical Pharmacology (12.2)]. Patients should also be advised to avoid tyramine-containing nutritional supplements. Patients should be instructed to immediately report the occurrence of the following acute symptoms: severe headache, neck stiffness, heart racing or palpitations, or other sudden or unusual symptoms.
Concomitant Medication: Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications, including herbals, because of a potential for dangerous interactions. Instruct patients not to take EMSAM with medication that is contraindicated or within two weeks of stopping such medication (5 weeks for fluoxetine). Contraindicated medication should not be started within two weeks of stopping EMSAM [see Contraindications (4)].
Psychomotor Performance: EMSAM has not been shown to impair psychomotor performance; however, any psychoactive drug may potentially impair judgment, thinking, or motor skills. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that EMSAM therapy does not impair their ability to engage in such activities.
Alcohol: Patients should be told that, although EMSAM has not been shown to increase the impairment of mental and motor skills caused by alcohol, the concomitant use of EMSAM and alcohol in depressed patients is not recommended.
Pediatrics: Advise patients that EMSAM must not be used in children less than 12 years of age because of an increased risk of severe increases in blood pressure. Also, patients should be advised that EMSAM is not recommended for use in pediatric patients ages 12 to 17 years [see Use in Specific Populations (8.4)].
Pregnancy: Advise the pregnant woman about the potential risk to the fetus [see Use in Specific Populations (8.1)].
Lactation: Advise a woman that breastfeeding is not recommended during treatment with EMSAM treatment and for 5 days after the final dose [see Use in Specific Populations (8.2)].
How to Use EMSAM
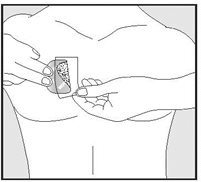
Detailed Instructions are provided in the Medication Guide. Prescribers should instruct patients on the following:
- •
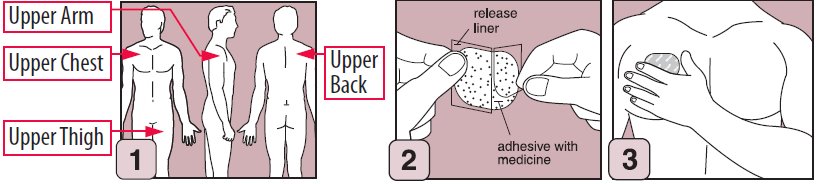
- EMSAM should be applied to dry, intact skin on the upper torso (below the neck and above the waist), upper thigh or the outer surface of the upper arm. A new application site should be selected with each new transdermal system to avoid re-application to the same site on consecutive days. Transdermal systems should be applied at approximately the same time each day.
- •
- Apply the transdermal system to an area of skin that is not hairy, oily, irritated, broken, scarred or calloused. Do not place the transdermal system where your clothing is tight, which could cause the transdermal system to rub off.
- •
- After you have selected the site for your transdermal system, wash the area gently and thoroughly with soap and warm water. Rinse until all soap is removed. Dry the area with a clean dry towel.
- •
- Just before you apply the transdermal system, remove it from the pouch by tearing at the notches (do not use scissors). Remove half of the release liner and throw it away. Try not to touch the exposed side (sticky side) of the transdermal system, because the medicine could come off on your fingers.
- •
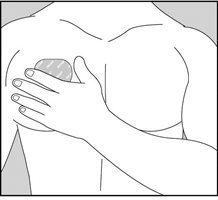
- Press the sticky side of the transdermal system firmly against the skin site that was just washed and dried. Remove the second half of the release liner and press the remaining sticky side firmly against your skin. Make sure that the transdermal system is flat against the skin (there should be no bumps or folds in the transdermal system) and is sticking securely. Be sure the edges are stuck to the skin surface.
- •
- After you have applied the transdermal system, wash your hands thoroughly with soap and water to remove any medicine that may have gotten on them. Do not touch your eyes until after you have washed your hands.
- •
- After 24 hours, remove your transdermal system slowly and carefully to avoid damaging your skin.
- •
- If the transdermal system is too sticky on your skin, and you need something to help you remove it:
- •
- Gently wash the area with warm water and mild soap.
- •
- A small amount of oil-based product (petroleum jelly, olive oil, or mineral oil) may be needed to help remove the transdermal system. Gently apply and spread the oil underneath the transdermal system edges.
- •
- Apply an oil-based product or lotion to your skin if any adhesive (glue) remains after you remove your transdermal system. This will gently loosen and remove any adhesive that is left over.
- •
- Fold the used EMSAM transdermal system in half and press it together firmly so that the sticky side sticks to itself.
- •
- Safely throw away the folded transdermal system in a container with a lid right away so that children and pets cannot reach it.
- •
- Safely throw away any unused EMSAM transdermal systems that are left over from the prescription as soon as they are no longer needed.
- •
- Wash your hands with soap and water.
- •
- If your transdermal system falls off, apply a new transdermal system to a new site and resume your previous schedule.
- •
- Only one EMSAM transdermal system should be worn at a time.
- •
- Do not cut the EMSAM transdermal system into smaller portions.
The brands listed are trademarks of their respective owners.
MEDICATION GUIDE
|
EMSAM® [EM sam]
|
||
|
What is the most important information I should know about EMSAM?
Call a healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you: |
||
|
|
|
|
3. EMSAM is not for children less than 12 years of age. EMSAM may cause a severe increase in blood pressure in children less than 12 years of age. |
||
|
What is EMSAM? EMSAM is a prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD). EMSAM belongs to the class of medicines known as monoamine oxidase inhibitors (MAOI). EMSAM is a transdermal system (patch) you apply to your skin. It is important to talk with your healthcare provider about the risks of treating depression and also the risk of not treating it. You should discuss all treatment choices with your healthcare provider. Talk to your healthcare provider if you do not think that your condition is getting better with EMSAM treatment. |
||
|
Who should not use EMSAM? Using EMSAM with certain antidepressants and certain pain, cold and cough symptom medicines may cause a potentially life-threatening problem called serotonin syndrome (See “What are the possible side effects of EMSAM?”). Do not use EMSAM if you:
Ask your healthcare provider or pharmacist if you are not sure if you take these medicines. |
||
|
Before you use EMSAM, tell your healthcare provider about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. EMSAM and some medicines may interact with each other, may not work as well, or may cause serious side effects when taken together. Especially tell your healthcare provider if you take:
Some of these medicines need to be stopped for up to 5 weeks before you can start using EMSAM and for 2 weeks after you stop using EMSAM. Ask your healthcare provider or pharmacist if you are not sure if you take these medicines. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine. |
||
|
How should I use EMSAM?
|
||
|
|
|
|
||
|
What should I avoid while using EMSAM?
|
||
|
Type of Food and Drink |
Foods and drinks you should avoid that contain Tyramine |
|
|
Meat, Poultry, and Fish |
|
|
|
Vegetables |
|
|
|
Dairy (milk products) |
|
|
|
Drinks |
|
|
|
Other |
|
|
|
||
|
What are the possible side effects of EMSAM? EMSAM may cause serious side effects, including:
|
||
|
|
|
|
If you suddenly have these symptoms, stop using EMSAM immediately by removing the patch and go to the nearest hospital emergency room right away.
|
||
|
|
|
|
||
|
|
|
|
The most common side effects of EMSAM include:
These are not all the possible side effects of EMSAM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store EMSAM?
|
||
|
General information about the safe and effective use of EMSAM. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EMSAM for a condition for which it was not prescribed. Do not give EMSAM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about EMSAM that is written for health professionals. |
||
|
What are the ingredients in EMSAM? Active Ingredient: selegiline Inactive Ingredients: acrylic adhesive, ethylene vinyl acetate, polyethylene, polyester, polyurethane, and silicone coated polyester Distributed by: Mylan Specialty L.P., Morgantown, WV 26505 U.S.A. Manufactured for: For more information, go to www.EMSAM.com or call 1-800-395-3376. |
||
This Medication Guide has been approved by the U.S. Food and Drug Administration
Issued: 5/2020
Instructions for Use
EMSAM® [EM sam]
(selegiline transdermal system)
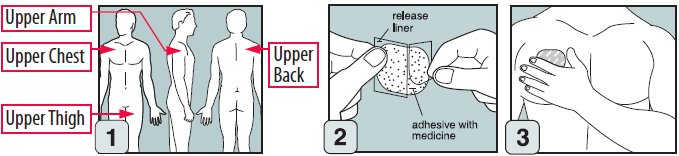
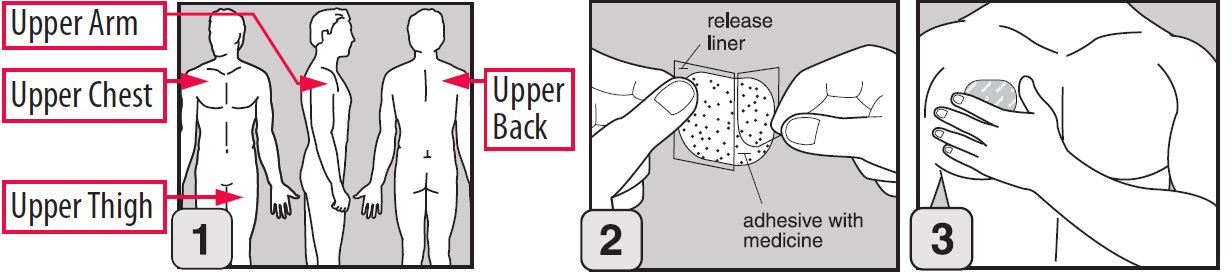
Step 1. Where to apply EMSAM
- •
- Place your EMSAM transdermal system (patch) on one of the following areas (sites) on your body. See Figure A.
- •
- EMSAM should be applied to dry, intact skin on the upper torso (below the neck and above the waist), upper thigh or the outer surface of the upper arm. Clothing and movement may make your patch rub off.
- •
- Choose a new site each time you change your patch. Do not use the same site 2 days in a row.
Step 2. Before you apply EMSAM
- •
- Make sure the area on your skin where you apply your patch:
- •
- is freshly washed with soap and warm water, then dried with a towel
- •
- does not have any powder, oil or lotion
- •
- does not have any cuts or irritation, including rashes, swelling, redness, or other skin problems
- •
- is not hairy, scarred or calloused
Step 3. How to apply EMSAM
- •
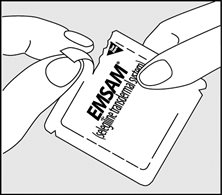
- Remove EMSAM from its sealed pouch by tearing at the notches (do not use scissors). Pull the pouch open. See Figure B.
- •
- Look at the patch to make sure it is not damaged. The patch should separate easily from the release liner. Throw away the patch if the release liner is hard to remove.
- •
- Do not keep or store your EMSAM outside of the sealed pouch. Do not cut your EMSAM into smaller pieces.
- •
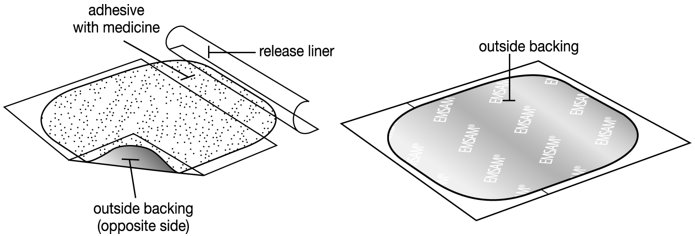
- The EMSAM patch has three layers. See Figures C and D.
- Layers:
-
- •
- Release liner: The release liner is the layer that you remove before you put the patch on. See Figure C.
- •
- Adhesive with medicine: The adhesive with medicine is the layer that sticks to your skin. See Figure C.
- •
- Outside backing: The outside backing is the layer you see after you put the patch on your skin. See Figure D.
- •
- Apply the patch right away after you remove the patch from its sealed pouch.
- •
- Hold the patch with the release liner facing you.
- •
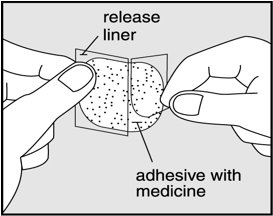
- Gently peel half of the release liner off the patch and throw it away. See Figure E.
- •
- Avoid touching the sticky side of the patch with your fingers. If you accidently touch the sticky side of the patch, wash your hands right away so the medicine does not go into the skin on your hands.
- •
- Using the other half of the release liner as a handle, apply the sticky side of the patch to your selected area. See Figure F.
- •
- Hold an edge of the remaining half of the release liner and slowly peel it off. See Figure G.
- •
- After the release liner is removed, there should not be any adhesive sticking to the liner.
- •
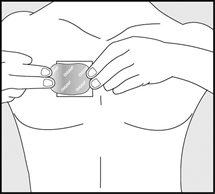
- Using your fingers and palm of your hand, press the entire patch firmly into place against your skin. See Figure H.
- •
- Make sure the patch firmly sticks to your skin.
- •
- Gently rub the edges of your patch with your fingers to make sure the patch sticks to your skin.
- •
- Wash your hands well with soap and water after you apply your patch to remove any medicine. Do not touch your eyes until after you have washed your hands.
- •
- If the patch becomes loose, press it back in place. If your EMSAM patch falls off, apply a new EMSAM patch to a new site and follow your normal schedule for changing patches.
- •
- If you forget to change your patch after 24 hours, remove the old patch. Put on a new patch in a different area and continue to follow your normal schedule for changing patches.
Step 4. Removing and disposing of your patch
- •
- After 24 hours, remove your patch slowly and carefully to avoid damaging your skin.
- •
- If the patch is too sticky on your skin and you need something to help you remove it:
- •
- Gently wash the area with warm water and mild soap.
- •
- A small amount of oil-based product (petroleum jelly, olive oil, or mineral oil) may be needed to help remove the patch. Gently apply and spread the oil underneath the patch edges.
- •
- Apply an oil-based product or lotion to your skin if any adhesive (glue) remains after you remove your patch. This will gently loosen and remove any adhesive that is left over.
- •
- If you still cannot easily remove the patch, ask your doctor or pharmacist about what to do for this problem.
- •
- Fold the used EMSAM patch in half and press it together firmly so that the sticky side sticks to itself.
- •
- Safely throw away the folded patch in a container with a lid right away so that children and pets cannot reach it.
- •
- Safely throw away any unused EMSAM patches that are left over from the prescription as soon as they are no longer needed.
- •
- To safely throw away the patches:
- •
- Remove the leftover patches from their protective pouches and remove the release liners.
- •
- Fold the patches in half with the sticky sides together and throw the patches away in a container with a lid.
- •
- Wash your hands with soap and water.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
DISTRIBUTED BY:
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
MANUFACTURED FOR:
Somerset Pharmaceuticals, Inc.
Morgantown, WV 26505 U.S.A.
Revised: 5/2020
EMSAM:PIR15/EMSAM:PLR15
PRINCIPAL DISPLAY PANEL - 6 mg/24 h
NDC-49502-900-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
EMSAM®
(selegiline transdermal system)
6 mg/24 h
FOR TRANSDERMAL USE ONLY
Patient: Read enclosed Medication Guide.
Pharmacist: Dispense with enclosed
Medication Guide.
30 TRANSDERMAL
SYSTEMS
INSTRUCTIONS FOR APPLICATION
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 6 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Mfd. for
Somerset Pharmaceuticals, Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S033:93:30C:R10
PRINCIPAL DISPLAY PANEL - 9 mg/24 h
NDC-49502-901-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
EMSAM®
(selegiline transdermal system)
9 mg/24 h
Important: Do not eat certain foods.
Read enclosed Medication Guide.
FOR TRANSDERMAL USE ONLY
Pharmacist: Dispense with enclosed
Medication Guide.
30 TRANSDERMAL
SYSTEMS
INSTRUCTIONS FOR APPLICATION
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 9 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Mfd. for
Somerset Pharmaceuticals, Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S044:93:30C:R10
PRINCIPAL DISPLAY PANEL - 12 mg/24 h
NDC-49502-902-30
EACH UNIT DOSE PACKAGE IS NOT CHILD RESISTANT
Rx only
EMSAM®
(selegiline transdermal system)
12 mg/24 h
Important: Do not eat certain foods.
Read enclosed Medication Guide.
FOR TRANSDERMAL USE ONLY
Pharmacist: Dispense with enclosed
Medication Guide.
30 TRANSDERMAL
SYSTEMS
INSTRUCTIONS FOR APPLICATION
1. Select and prepare an appropriate site on the upper chest or back (below the neck and above the waist), the upper thigh, or the outer surface of the upper arm, as directed in the enclosed Medication Guide.
2. Bend both sides of the clear peelable liner. Peel off one strip only of the clear liner. Avoid touching the sticky side of the transdermal system.
3. Apply sticky side of the transdermal system to the chosen skin site. Remove remaining strip, gently roll remaining part onto skin, and press transdermal system firmly in place with the tips of your fingers.
For more detailed instructions, read enclosed Medication Guide.
Dosage: Apply one (1) transdermal system every 24 hours. Read enclosed Medication Guide.
Each transdermal system has a release rate of 12 mg selegiline per 24 hour period. Wear only one transdermal system at a time.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dist. by
Mylan Specialty L.P.
Morgantown, WV 26505 U.S.A.
Mfd. for
Somerset Pharmaceuticals, Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S055:93:30C:R10