WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
• Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use (see WARNINGS).
• NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and/ NAPROXEN SODIUM DS are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see CONTRAINDICATIONS, WARNINGS).
Gastrointestinal Bleeding, Ulceration, and Perforation
• NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (see WARNINGS).
DESCRIPTION
Naproxen is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs.
The chemical names for naproxen and naproxen sodium are (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid and (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid, sodium salt, respectively. Naproxen and naproxen sodium have the following structures, respectively:

Naproxen has a molecular weight of 230.26 and a molecular formula of C14H14O3. Naproxen sodium has a molecular weight of 252.23 and a molecular formula of C14H13NaO3.
Naproxen is an odorless, white to off-white crystalline substance. It is lipid-soluble, practically insoluble in water at low pH and freely soluble in water at high pH. The octanol/water partition coefficient of naproxen at pH 7.4 is 1.6 to 1.8. Naproxen sodium is a white to creamy white, crystalline solid, freely soluble in water at neutral pH.
NAPROXEN (naproxen tablets) is available as yellow tablets containing 250 mg of naproxen, pink tablets containing 375 mg of naproxen and yellow tablets containing 500 mg of naproxen for oral administration. The inactive ingredients are croscarmellose sodium, iron oxides, povidone and magnesium stearate.
EC-NAPROXEN (naproxen delayed-release tablets) is available as enteric-coated white tablets containing 375 mg of naproxen and 500 mg of naproxen for oral administration. The inactive ingredients are croscarmellose sodium, povidone and magnesium stearate. The enteric coating dispersion contains methacrylic acid copolymer, talc, triethyl citrate, sodium hydroxide and purified water. The dissolution of this enteric-coated naproxen tablet is pH dependent with rapid dissolution above pH 6. There is no dissolution below pH 4.
NAPROXEN SODIUM (naproxen sodium tablets) is available as blue tablets containing 275 mg of naproxen sodium and NAPROXEN SODIUM DS (naproxen sodium tablets) is available as dark blue tablets containing 550 mg of naproxen sodium for oral administration. The inactive ingredients are magnesium stearate, microcrystalline cellulose, povidone and talc. The coating suspension for the NAPROXEN SODIUM 275 mg tablet may contain hydroxypropyl methylcellulose 2910, Opaspray K-1-4210A, polyethylene glycol 8000 or Opadry YS-1-4215. The coating suspension for the NAPROXEN SODIUM DS 550 mg tablet may contain hydroxypropyl methylcellulose 2910, Opaspray K-1-4227, polyethylene glycol 8000 or Opadry YS-1-4216.
CLINICAL PHARMACOLOGY
Mechanism of Action
Naproxen has analgesic, anti-inflammatory, and antipyretic properties. The sodium salt of naproxen has been developed as a more rapidly absorbed formulation of naproxen for use as an analgesic.
The mechanism of action of the naproxen, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Naproxen is a potent inhibitor of prostaglandin synthesis in vitro. Naproxen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because naproxen is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
Pharmacokinetics
Naproxen and naproxen sodium are rapidly and completely absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%. The different dosage forms of NAPROXEN are bioequivalent in terms of extent of absorption (AUC) and peak concentration (Cmax); however, the products do differ in their pattern of absorption. These differences between naproxen products are related to both the chemical form of naproxen used and its formulation. Even with the observed differences in pattern of absorption, the elimination half-life of naproxen is unchanged across products ranging from 12 to 17 hours. Steady-state levels of naproxen are reached in 4 to 5 days, and the degree of naproxen accumulation is consistent with this half-life. This suggests that the differences in pattern of release play only a negligible role in the attainment of steady-state plasma levels.
Absorption
NAPROXEN
After administration of NAPROXEN tablets, peak plasma levels are attained in 2 to 4 hours. After oral administration of ANAPROX, peak plasma levels are attained in 1 to 2 hours. The difference in rates between the two products is due to the increased aqueous solubility of the sodium salt of naproxen used in ANAPROX. Peak plasma levels of naproxen given as NAPROXEN Suspension are attained in 1 to 4 hours.
EC-NAPROXEN
EC-NAPROXEN is designed with a pH-sensitive coating to provide a barrier to disintegration in the acidic environment of the stomach and to lose integrity in the more neutral environment of the small intestine. The enteric polymer coating selected for EC-NAPROXEN dissolves above pH 6. When EC-NAPROXEN was given to fasted subjects, peak plasma levels were attained about 4 to 6 hours following the first dose (range: 2 to 12 hours). An in vivo study in man using radiolabeled EC-NAPROXEN tablets demonstrated that EC-NAPROXEN dissolves primarily in the small intestine rather than in the stomach, so the absorption of the drug is delayed until the stomach is emptied.
When EC-NAPROXEN and NAPROXEN were given to fasted subjects (n=24) in a crossover study following 1 week of dosing, differences in time to peak plasma levels (Tmax) were observed, but there were no differences in total absorption as measured by Cmax and AUC:
| EC-NAPROXEN*
500 mg bid | EC-NAPROXEN*
500 mg bid |
|
| Cmax (µg/mL) | 94.9 (18%) | 97.4 (13%) |
| Tmax (hours) | 4 (39%) | 1.9 (61%) |
| AUC0–12 hr (µg·hr/mL) | 845 (20%) | 767 (15%) |
*Mean value (coefficient of variation)
Antacid Effects
When EC-NAPROXEN was given as a single dose with antacid (54 mEq buffering capacity), the peak plasma levels of naproxen were unchanged, but the time to peak was reduced (mean Tmax fasted 5.6 hours, mean Tmax with antacid 5 hours), although not significantly. (see PRECAUTIONS; Drug Interactions).
Food Effects
When EC-NAPROXEN was given as a single dose with food, peak plasma levels in most subjects were achieved in about 12 hours (range: 4 to 24 hours). Residence time in the small intestine until disintegration was independent of food intake. The presence of food prolonged the time the tablets remained in the stomach, time to first detectable serum naproxen levels, and time to maximal naproxen levels (Tmax), but did not affect peak naproxen levels (Cmax).
Distribution
Naproxen has a volume of distribution of 0.16 L/kg. At therapeutic levels naproxen is greater than 99% albumin-bound. At doses of naproxen greater than 500 mg/day there is less than proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough Css 36.5, 49.2 and 56.4 mg/L with 500, 1000 and 1500 mg daily doses of naproxen, respectively). The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma (see PRECAUTIONS: Nursing Mothers).
Elimination
Metabolism
Naproxen is extensively metabolized in the liver to 6-0-desmethyl naproxen, and both parent and metabolites do not induce metabolizing enzymes. Both naproxen and 6-0-desmethyl naproxen are further metabolized to their respective acylglucuronide conjugated metabolites.
Excretion
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (<1%), 6-0-desmethyl naproxen (<1%) or their conjugates (66% to 92%). The plasma half-life of the naproxen anion in humans ranges from 12 to 17 hours. The corresponding half-lives of both naproxen’s metabolites and conjugates are shorter than 12 hours, and their rates of excretion have been found to coincide closely with the rate of naproxen disappearance from the plasma. Small amounts, 3% or less of the administered dose, are excreted in the feces. In patients with renal failure metabolites may accumulate (see WARNINGS; Renal Toxicity and Hyperkalemia).
Special Populations
Pediatric Patients
In pediatric patients aged 5 to 16 years with arthritis, plasma naproxen levels following a 5 mg/kg single dose of naproxen suspension (see DOSAGE AND ADMINISTRATION) were found to be similar to those found in normal adults following a 500 mg dose. The terminal half-life appears to be similar in pediatric and adult patients. Pharmacokinetic studies of naproxen were not performed in pediatric patients younger than 5 years of age. Pharmacokinetic parameters appear to be similar following administration of naproxen suspension or tablets in pediatric patients. EC-NAPROXEN has not been studied in subjects under the age of 18.
Geriatric Patients
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly, although the unbound fraction is <1% of the total naproxen concentration. Unbound trough naproxen concentrations in elderly subjects have been reported to range from 0.12% to 0.19% of total naproxen concentration, compared with 0.05% to 0.075% in younger subjects. The clinical significance of this finding is unclear, although it is possible that the increase in free naproxen concentration could be associated with an increase in the rate of adverse events per a given dosage in some elderly patients.
Race
Pharmacokinetic differences due to race have not been studied.
Hepatic Impairment
Naproxen pharmacokinetics has not been determined in subjects with hepatic insufficiency.
Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose.
Renal Impairment
Naproxen pharmacokinetics has not been determined in subjects with renal insufficiency. Given that naproxen, its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen metabolites to accumulate in the presence of renal insufficiency. Elimination of naproxen is decreased in patients with severe renal impairment. Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance <30 mL/min) (see WARNINGS; Renal Toxicity and Hyperkalemia).
Drug Interaction Studies
Aspirin: When NSAIDs were administered with aspirin, the protein binding of NSAIDs were reduced, although the clearance of free NSAID was not altered. The clinical significance of this interaction is not known. See Table 1 for clinically significant drug interactions of NSAIDs with aspirin (see PRECAUTIONS: Drug Interactions).
CLINICAL STUDIES
General Information
Naproxen has been studied in patients with rheumatoid arthritis, osteoarthritis, juvenile arthritis, ankylosing spondylitis, tendonitis and bursitis, and acute gout. Improvement in patients treated for rheumatoid arthritis was demonstrated by a reduction in joint swelling, a reduction in duration of morning stiffness, a reduction in disease activity as assessed by both the investigator and patient, and by increased mobility as demonstrated by a reduction in walking time. Generally, response to naproxen has not been found to be dependent on age, sex, severity or duration of rheumatoid arthritis.
In patients with osteoarthritis, the therapeutic action of naproxen has been shown by a reduction in joint pain or tenderness, an increase in range of motion in knee joints, increased mobility as demonstrated by a reduction in walking time, and improvement in capacity to perform activities of daily living impaired by the disease.
In a clinical trial comparing standard formulations of naproxen 375 mg bid (750 mg a day) vs 750 mg bid (1500 mg/day), 9 patients in the 750 mg group terminated prematurely because of adverse events. Nineteen patients in the 1500 mg group terminated prematurely because of adverse events. Most of these adverse events were gastrointestinal events.
In clinical studies in patients with rheumatoid arthritis, osteoarthritis, and juvenile arthritis, naproxen has been shown to be comparable to aspirin and indomethacin in controlling the aforementioned measures of disease activity, but the frequency and severity of the milder gastrointestinal adverse effects (nausea, dyspepsia, heartburn) and nervous system adverse effects (tinnitus, dizziness, lightheadedness) were less in naproxen-treated patients than in those treated with aspirin or indomethacin.
In patients with ankylosing spondylitis, naproxen has been shown to decrease night pain, morning stiffness and pain at rest. In double-blind studies the drug was shown to be as effective as aspirin, but with fewer side effects.
In patients with acute gout, a favorable response to naproxen was shown by significant clearing of inflammatory changes (eg, decrease in swelling, heat) within 24 to 48 hours, as well as by relief of pain and tenderness.
Naproxen has been studied in patients with mild to moderate pain secondary to postoperative, orthopedic, postpartum episiotomy and uterine contraction pain and dysmenorrhea. Onset of pain relief can begin within 1 hour in patients taking naproxen and within 30 minutes in patients taking naproxen sodium. Analgesic effect was shown by such measures as reduction of pain intensity scores, increase in pain relief scores, decrease in numbers of patients requiring additional analgesic medication, and delay in time to remedication. The analgesic effect has been found to last for up to 12 hours.
Naproxen may be used safely in combination with gold salts and/or corticosteroids; however, in controlled clinical trials, when added to the regimen of patients receiving corticosteroids, it did not appear to cause greater improvement over that seen with corticosteroids alone. Whether naproxen has a “steroid-sparing” effect has not been adequately studied. When added to the regimen of patients receiving gold salts, naproxen did result in greater improvement. Its use in combination with salicylates is not recommended because there is evidence that aspirin increases the rate of excretion of naproxen and data are inadequate to demonstrate that naproxen and aspirin produce greater improvement over that achieved with aspirin alone. In addition, as with other NSAIDs, the combination may result in higher frequency of adverse events than demonstrated for either product alone.
In 51Cr blood loss and gastroscopy studies with normal volunteers, daily administration of 1000 mg of naproxen as 1000 mg of NAPROXEN or 1100 mg of naproxen sodium has been demonstrated to cause statistically significantly less gastric bleeding and erosion than 3250 mg of aspirin.
Three 6-week, double-blind, multicenter studies with EC-NAPROXEN (naproxen) (375 or 500 mg bid, n=385) and NAPROXEN (375 or 500 mg bid, n=279) were conducted comparing EC-NAPROXEN with NAPROXEN, including 355 rheumatoid arthritis and osteoarthritis patients who had a recent history of NSAID-related GI symptoms. These studies indicated that EC-NAPROXEN and NAPROXEN showed no significant differences in efficacy or safety and had similar prevalence of minor GI complaints. Individual patients, however, may find one formulation preferable to the other.
Five hundred and fifty-three patients received EC-NAPROXEN during long-term open-label trials (mean length of treatment was 159 days). The rates for clinically-diagnosed peptic ulcers and GI bleeds were similar to what has been historically reported for long-term NSAID use.
Geriatric Patients
The hepatic and renal tolerability of long-term naproxen administration was studied in two double-blind clinical trials involving 586 patients. Of the patients studied, 98 patients were age 65 and older and 10 of the 98 patients were age 75 and older. Naproxen was administered at doses of 375 mg twice daily or 750 mg twice daily for up to 6 months. Transient abnormalities of laboratory tests assessing hepatic and renal function were noted in some patients, although there were no differences noted in the occurrence of abnormal values among different age groups.
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of NAPROXEN, EC-NAPROXEN, ANAPROX, ANAPROX DS or NAPROXEN Suspension and other treatment options before deciding to use NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation).
Naproxen as NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is indicated:
- For the relief of the signs and symptoms of rheumatoid arthritis
- For the relief of the signs and symptoms of osteoarthritis
- For the relief of the signs and symptoms of ankylosing spondylitis
- For the relief of the signs and symptoms of juvenile arthritis
Naproxen as NAPROXEN Suspension is recommended for juvenile rheumatoid arthritis in order to obtain the maximum dosage flexibility based on the patient’s weight.
Naproxen as NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS is also indicated:
- For relief of the signs and symptoms of tendonitis
- For relief of the signs and symptoms of bursitis
- For relief of the signs and symptoms of acute gout
- For the management of pain
- For the management of primary dysmenorrhea
EC-NAPROXEN is not recommended for initial treatment of acute pain because the absorption of naproxen is delayed compared to absorption from other naproxen-containing products (see CLINICAL PHARMACOLOGY, DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS are contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to naproxen, naproxen sodium, or any components of the drug product (see WARNINGS; Anaphylactic Reactions, Serious Skin Reactions).
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients (see WARNINGS; Exacerbation of Asthma Related to Aspirin Sensitivity).
- In the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS; Cardiovascular Thrombotic Events).
WARNINGS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as naproxen, increases the risk of serious gastrointestinal (GI) events (see WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation).
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG (see CONTRAINDICATIONS).
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post-MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
Gastrointestinal Bleeding, Ulceration, and Perforation
NSAIDs, including naproxen cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3-6 months, and in about 2%-4% of patients treated for one year. However, even short-term NSAID therapy is not without risk.
Risk Factors for GI Bleeding, Ulceration, and Perforation
Patients with a prior history of peptic ulcer disease and/or GI bleeding who used NSAIDs had a greater than 10-fold increased risk for developing a GI bleed compared to patients without these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include longer duration of NSAID therapy; concomitant use of oral corticosteroids, aspirin, anticoagulants, or selective serotonin reuptake inhibitors (SSRIs); smoking; use of alcohol; older age; and poor general health status. Most postmarketing reports of fatal GI events occurred in elderly or debilitated patients. Additionally, patients with advanced liver disease and/or coagulopathy are at increased risk for GI bleeding.
Strategies to Minimize the GI Risks in NSAID-treated patients:
- Use the lowest effective dosage for the shortest possible duration.
- Avoid administration of more than one NSAID at a time.
- Avoid use in patients at higher risk unless benefits are expected to outweigh the increased risk of bleeding. For such patients, as well as those with active GI bleeding, consider alternate therapies other than NSAIDs.
- Remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy.
- If a serious GI adverse event is suspected, promptly initiate evaluation and treatment, and discontinue NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS until a serious GI adverse event is ruled out.
- In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, monitor patients more closely for evidence of GI bleeding (see PRECAUTIONS; Drug Interactions).
Hepatotoxicity
Elevations of ALT or AST (three or more times the upper limit of normal [ULN]) have been reported in approximately 1% of patients in clinical trials. In addition, rare, sometimes fatal, cases of severe hepatic injury, including fulminant hepatitis, liver necrosis and hepatic failure have been reported.
Elevations of ALT or AST (less than three times ULN) may occur in up to 15% of patients taking NSAIDs including naproxen.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS immediately, and perform a clinical evaluation of the patient.
Hypertension
NSAIDs, including NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS, can lead to new onset of hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs (see PRECAUTIONS; Drug Interactions).
Monitor blood pressure (BP) during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists’ Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalization for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of naproxen may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) (see PRECAUTIONS; Drug Interactions).
Avoid the use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Since each NAPROXEN SODIUM or NAPROXEN SODIUM DS tablet contains 25 mg or 50 mg of sodium (about 1 mEq per each 250 mg of naproxen), this should be considered in patients whose overall intake of sodium must be severely restricted.
Renal Toxicity and Hyperkalemia
Renal Toxicity
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury.
Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors or ARBs, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
No information is available from controlled clinical studies regarding the use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS in patients with advanced renal disease. The renal effects of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS may hasten the progression of renal dysfunction in patients with preexisting renal disease.
Correct volume status in dehydrated or hypovolemic patients prior to initiating NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS. Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia during use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS (see PRECAUTIONS; Drug Interactions). Avoid the use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS in patients with advanced renal disease unless the benefits are expected to outweigh the risk of worsening renal function. If NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is used in patients with advanced renal disease, monitor patients for signs of worsening renal function.
Hyperkalemia
Increases in serum potassium concentration, including hyperkalemia, have been reported with use of NSAIDs, even in some patients without renal impairment. In patients with normal renal function, these effects have been attributed to a hyporeninemic-hypoaldosteronism state.
Anaphylactic Reactions
Naproxen has been associated with anaphylactic reactions in patients with and without known hypersensitivity to naproxen and in patients with aspirin-sensitive asthma (see CONTRAINDICATIONS, WARNINGS; Exacerbation of Asthma Related to Aspirin Sensitivity).
Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS are contraindicated in patients with this form of aspirin sensitivity (see CONTRAINDICATIONS). When NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS are used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
Serious Skin Reactions
NSAIDs, including naproxen, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions and to discontinue the use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS at the first appearance of skin rash or any other sign of hypersensitivity. NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS are contraindicated in patients with previous serious skin reactions to NSAIDs (see CONTRAINDICATIONS).
Premature Closure of Fetal Ductus Arteriosus
Naproxen may cause premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs, including NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS, in pregnant women starting at 30 weeks of gestation (third trimester) (see PRECAUTIONS; Pregnancy).
Hematologic Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross blood loss, fluid retention, or an incompletely described effect on erythropoiesis. If a patient treated with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS has any signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders, or concomitant use of warfarin and other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding (see PRECAUTIONS; Drug Interactions).
PRECAUTIONS
General
Naproxen-containing products such as NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS, and other naproxen products should not be used concomitantly since they all circulate in the plasma as the naproxen anion.
NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids and the patient should be observed closely for any evidence of adverse effects, including adrenal insufficiency and exacerbation of symptoms of arthritis.
Patients with initial hemoglobin values of 10 g or less who are to receive long-term therapy should have hemoglobin values determined periodically.
Because of adverse eye findings in animal studies with drugs of this class, it is recommended that ophthalmic studies be carried out if any change or disturbance in vision occurs.
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately (see WARNINGS; Cardiovascular Thrombotic Events).
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for the signs and symptoms of GI bleeding (see WARNINGS; Gastrointestinal Bleeding, Ulceration, and Perforation).
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, instruct patients to stop NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and seek immediate medical therapy (see WARNINGS; Hepatotoxicity).
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur (see WARNINGS; Heart Failure and Edema).
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur (see CONTRAINDICATION, WARNINGS; Anaphylactic Reactions).
Serious Skin Reactions
Advise patients to stop NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS immediately if they develop any type of rash and to contact their healthcare provider as soon as possible (see WARNINGS; Serious Skin Reactions).
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including VOLTAREN, may be associated with a reversible delay in ovulation (see PRECAUTIONS; Carcinogenesis, Mutagenesis, Impairment of Fertility).
Fetal Toxicity
Inform pregnant women to avoid use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM, NAPROXEN SODIUM DS and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus (see WARNINGS; Premature Closure of Fetal Ductus Arteriosus).
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy (see WARNINGS;: Gastrointestinal Bleeding, Ulceration, and Perforation, PRECAUTIONS; Drug Interactions). Alert patients that NSAIDs may be present in “over the counter” medications for treatment of colds, fever, or insomnia.
Use of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS until they talk to their healthcare provider (see PRECAUTIONS; Drug Interactions).
Activities Requiring Alertness
Caution should be exercised by patients whose activities require alertness if they experience drowsiness, dizziness, vertigo or depression during therapy with naproxen.
Masking of Inflammation and Fever
The pharmacological activity of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and a chemistry profile periodically (see WARNINGS; Gastrointestinal Bleeding, Ulceration, and Perforation, and Hepatotoxicity).
Drug Interactions
See Table 1 for clinically significant drug interactions with naproxen.
| Drugs That Interfere with Hemostasis | |
| Clinical Impact: | • Naproxen and anticoagulants such as warfarin have a synergistic effect on bleeding. The concomitant use of naproxen and anticoagulants has an increased risk of serious bleeding compared to the use of either drug alone. • Serotonin release by platelets plays an important role in hemostasis. Case-control and cohort epidemiological studies showed that concomitant use of drugs that interfere with serotonin reuptake and an NSAID may potentiate the risk of bleeding more than an NSAID alone. Monitor patients with concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding (see WARNINGS; Hematologic Toxicity). |
| Intervention: | Monitor patients with concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding (see WARNINGS; Hematologic Toxicity). |
| Aspirin | |
| Clinical Impact: | Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone (see WARNINGS; Gastrointestinal Bleeding, Ulceration, and Perforation). |
| Intervention: | Concomitant use of NAPROXEN,
EC- NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding (see WARNINGS; Hematologic Toxicity). NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is not a substitute for low dose aspirin for cardiovascular protection. |
| ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers | |
| Clinical Impact: | • NSAIDs may diminish the antihypertensive effect of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers (including propranolol). • In patients who are elderly, volume-depleted (including those on diuretic therapy), or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. |
| Intervention: | • During concomitant use of NAPROXEN,
EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and ACE-inhibitors, ARBs, or beta-blockers, monitor blood pressure to ensure that the desired blood pressure is obtained. • During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and ACE-inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor for signs of worsening renal function (see WARNINGS; Renal Toxicity and Hyperkalemia). When these drugs are administered concomitantly, patients should be adequately hydrated. Assess renal function at the beginning of the concomitant treatment and periodically thereafter. |
| Diuretics | |
| Clinical Impact: | Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects (see WARNINGS; Renal Toxicity and Hyperkalemia). |
| Digoxin | |
| Clinical Impact: | The concomitant use of naproxen with digoxin has been reported to increase the serum and prolong the half-life of digoxin. |
| Intervention: | During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and digoxin, monitor serum digoxin levels. |
| Lithium | |
| Clinical Impact: | NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and lithium, monitor patients for signs of lithium toxicity. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). |
| Intervention: | During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact: | Concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUMDS and cyclosporine may increase cyclosporine’s nephrotoxicity. |
| Intervention: | During concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and cyclosporine, monitor patients for signs of worsening renal function. |
| NSAIDs and Salicylates | |
| Clinical Impact: | Concomitant use of naproxen with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy (see WARNINGS; Gastrointestinal Bleeding, Ulceration and Perforation). |
| Intervention: | The concomitant use of naproxen with other NSAIDs or salicylates is not recommended. |
| Pemetrexed | |
| Clinical Impact: | Concomitant use of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention: | During concomitant use of NAPROXEN,
EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed. In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. |
| Antacids and Sucralfate | |
| Clinical Impact: | Concomitant administration of some antacids (magnesium oxide or aluminum hydroxide) and sucralfate can delay the absorption of naproxen. |
| Intervention: | Concomitant administration of antacids such as magnesium oxide or aluminum hydroxide, and sucralfate with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is not recommended. Due to the gastric pH elevating effects of H2-blockers, sucralfate and intensive antacid therapy, concomitant administration of EC-NAPROXEN is not recommended. |
| Cholestyramine | |
| Clinical Impact: | Concomitant administration of cholestyramine can delay the absorption of naproxen. |
| Intervention: | Concomitant administration of cholestyramine with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS is not recommended. |
| Probenecid | |
| Clinical Impact: | Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly. |
| Intervention: | Patients simultaneously receiving NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and probenecid should be observed for adjustment of dose if required. |
| Other albumin-bound drugs | |
| Clinical Impact: | Naproxen is highly bound to plasma albumin; it thus has a theoretical potential for interaction with other albumin-bound drugs such as coumarin-type anticoagulants, sulphonylureas, hydantoins, other NSAIDs, and aspirin. |
| Intervention: | Patients simultaneously receiving NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS and a hydantoin, sulphonamide or sulphonylurea should be observed for adjustment of dose if required. |
| Bleeding times | |
| Clinical Impact: | Naproxen may decrease platelet aggregation and prolong bleeding time. |
| Intervention: | This effect should be kept in mind when bleeding times are determined. |
| Porter-Silber test | |
| Clinical Impact: | The administration of naproxen may result in increased urinary values for 17-ketogenic steroids because of an interaction between the drug and/or its metabolites with m-di-nitrobenzene used in this assay. |
| Intervention: | Although 17-hydroxy-corticosteroid measurements (Porter-Silber test) do not appear to be artifactually altered, it is suggested that therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used. |
| Urinary assays of 5-hydroxy indoleacetic acid (5HIAA) | |
| Clinical Impact: | Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA). |
| Intervention: | This effect should be kept in mind when urinary 5-hydroxy indoleacetic acid is determined. |
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A 2-year study was performed in rats to evaluate the carcinogenic potential of naproxen at rat doses t of 8, 16, and 24 mg/kg/day (0.05, 0.1, and 0.16 times the maximum recommended human daily dose [MRHD] of 1500 mg/day based on a body surface area comparison). No evidence of tumorigenicity was found.
Mutagenesis
Studies to evaluate the mutagenic potential of Naproxen, EC-Naproxen, Naproxen Sodium or Naproxen Sodium DS tablets have not been completed.
Impairment of fertility
Male rats were treated with 2, 5, 10, and 20 mg/kg naproxen by oral gavage for 60 days prior to mating and female rats were treated with the same doses for 14 days prior to mating and for the first 7 days of pregnancy. There were no adverse effects on fertility noted (up to 0.13 times the MRDH based on body surface area).
Pregnancy
Risk Summary
Use of NSAIDs, including Naproxen, EC-Naproxen, Naproxen Sodium, and Naproxen Sodium DS tablets, during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs, including Naproxen, in pregnant women starting at 30 weeks of gestation (third trimester) (see WARNINGS; Premature Closure of Fetal Ductus Arteriosus).
There are no adequate and well-controlled studies of Naproxen, EC-Naproxen, Naproxen Sodium, or Naproxen Sodium DS tablets in pregnant women.
Data from observational studies regarding potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In the general U.S. population, all clinically recognized pregnancies, regardless of drug exposure, have a background rate of 2-4% for major malformations, and 15-20% for pregnancy loss. In animal reproduction studies in rats, rabbit, and mice no evidence of teratogenicity or fetal harm when naproxen was administered during the period of organogenesis at doses 0.13, 0.26, and 0.6 times the maximum recommended human daily dose of 1500 mg/day, respectively. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as naproxen, resulted in increased pre- and post-implantation loss.
Data
Human Data
There is some evidence to suggest that when inhibitors of prostaglandin synthesis are used to delay preterm labor there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction and abnormal prostaglandin E levels in preterm infants. Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly starting at 30-weeks of gestation, or third trimester) should be avoided.
Animal Data
Reproduction studies have been performed in rats at 20 mg/kg/day (0.13 times the maximum recommended human daily dose of 1500 mg/day based on body surface area comparison), rabbits at 20 mg/kg/day (0.26 times the maximum recommended human daily dose, based on body surface area comparison), and mice at 170 mg/kg/day (0.6 times the maximum recommended human daily dose based on body surface area comparison) with no evidence of impaired fertility or harm to the fetus due to the drug. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as naproxen, resulted in increased pre- and post-implantation loss.
Labor and Delivery
There are no studies on the effects of Naproxen, EC-Naproxen, Naproxen Sodium or Naproxen Sodium DS tablets during labor or delivery. In animal studies, NSAIDS, including naproxen, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth.
Nursing Mothers
The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Naproxen, EC-Naproxen, Naproxen Sodium, or Naproxen Sodium DS tablets and any potential adverse effects on the breastfed infant from the Naproxen, EC-Naproxen, Naproxen Sodium, or Naproxen Sodium DS tablets or from the underlying maternal condition.
Females and Males of Reproductive Potential
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including Naproxen, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation.
Consider withdrawal of NSAIDs, including Naproxen, EC-Naproxen, Naproxen Sodium and Naproxen Sodium DS tablets, in women who have difficulties conceiving or who are undergoing investigation of infertility.
Pediatric Use
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established. Pediatric dosing recommendations for juvenile arthritis are based on well-controlled studies (see DOSAGE AND ADMINISTRATION). There are no adequate effectiveness or dose-response data for other pediatric conditions, but the experience in juvenile arthritis and other use experience have established that single doses of 2.5 to 5 mg/kg (as naproxen suspension, see DOSAGE AND ADMINISTRATION), with total daily dose not exceeding 15 mg/kg/day, are well tolerated in pediatric patients over 2 years of age.
Geriatric Use
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects (see WARNINGS; Cardiovascular Thrombotic Events, Gastrointestinal Bleeding, Ulceration, and Perforation, Hepatotoxicity, Renal Toxicity and Hyperkalemia, PRECAUTIONS; Laboratory Monitoring).
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
Experience indicates that geriatric patients may be particularly sensitive to certain adverse effects of nonsteroidal anti-inflammatory drugs. Elderly or debilitated patients seem to tolerate peptic ulceration or bleeding less well when these events do occur. Most spontaneous reports of fatal GI events are in the geriatric population (see WARNINGS; Gastrointestinal Bleeding, Ulceration, and Perforation).
Naproxen is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Geriatric patients may be at a greater risk for the development of a form of renal toxicity precipitated by reduced prostaglandin formation during administration of nonsteroidal anti-inflammatory drugs (see WARNINGS: Renal Toxicity and Hyperkalemia).
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events (see WARNINGS)
- GI Bleeding, Ulceration and Perforation (see WARNINGS)
- Hepatotoxicity (see WARNINGS)
- Hypertension (see WARNINGS)
- Heart Failure and Edema (see WARNINGS)
- Renal Toxicity and Hyperkalemia (see WARNINGS)
- Anaphylactic Reactions (see WARNINGS)
- Serious Skin Reactions (see WARNINGS)
- Hematologic Toxicity (see WARNINGS)
Adverse reactions reported in controlled clinical trials in 960 patients treated for rheumatoid arthritis or osteoarthritis are listed below. In general, reactions in patients treated chronically were reported 2 to 10 times more frequently than they were in short-term studies in the 962 patients treated for mild to moderate pain or for dysmenorrhea. The most frequent complaints reported related to the gastrointestinal tract.
A clinical study found gastrointestinal reactions to be more frequent and more severe in rheumatoid arthritis patients taking daily doses of 1500 mg naproxen compared to those taking 750 mg naproxen (see CLINICAL PHARMACOLOGY).
In controlled clinical trials with about 80 pediatric patients and in well-monitored, open-label studies with about 400 pediatric patients with juvenile arthritis treated with naproxen, the incidence of rash and prolonged bleeding times were increased, the incidence of gastrointestinal and central nervous system reactions were about the same, and the incidence of other reactions were lower in pediatric patients than in adults.
In patients taking naproxen in clinical trials, the most frequently reported adverse experiences in approximately 1% to 10% of patients are:
Gastrointestinal (GI) Experiences, including: heartburn*, abdominal pain*, nausea*, constipation*, diarrhea, dyspepsia, stomatitis
Central Nervous System: headache*, dizziness*, drowsiness*, lightheadedness, vertigo
Dermatologic: pruritus (itching)*, skin eruptions*, ecchymoses*, sweating, purpura
Special Senses: tinnitus*, visual disturbances, hearing disturbances
Cardiovascular: edema*, palpitations
General: dyspnea*, thirst
*Incidence of reported reaction between 3% and 9%. Those reactions occurring in less than 3% of the patients are unmarked.
In patients taking NSAIDs, the following adverse experiences have also been reported in approximately 1% to 10% of patients.
Gastrointestinal (GI) Experiences, including: flatulence, gross bleeding/perforation, GI ulcers (gastric/duodenal), vomiting
General: abnormal renal function, anemia, elevated liver enzymes, increased bleeding time, rashes
The following are additional adverse experiences reported in <1% of patients taking naproxen during clinical trials and through postmarketing reports. Those adverse reactions observed through postmarketing reports are italicized.
Body as a Whole: anaphylactoid reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever)
Cardiovascular: congestive heart failure, vasculitis, hypertension, pulmonary edema
Gastrointestinal: inflammation, bleeding (sometimes fatal, particularly in the elderly), ulceration, perforation and obstruction of the upper or lower gastrointestinal tract. Esophagitis, stomatitis, hematemesis, pancreatitis, vomiting, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn’s disease).
Hepatobiliary: jaundice, abnormal liver function tests, hepatitis (some cases have been fatal)
Hemic and Lymphatic: eosinophilia, leucopenia, melena, thrombocytopenia, agranulocytosis, granulocytopenia, hemolytic anemia, aplastic anemia
Metabolic and Nutritional: hyperglycemia, hypoglycemia
Nervous System: inability to concentrate, depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions
Respiratory: eosinophilic pneumonitis, asthma
Dermatologic: alopecia, urticaria, skin rashes, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens-Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored.
Special Senses: hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema
Urogenital: glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine
Reproduction (female): infertility
In patients taking NSAIDs, the following adverse experiences have also been reported in <1% of patients.
Body as a Whole: fever, infection, sepsis, anaphylactic reactions, appetite changes, death
Cardiovascular: hypertension, tachycardia, syncope, arrhythmia, hypotension, myocardial infarction
Gastrointestinal: dry mouth, esophagitis, gastric/peptic ulcers, gastritis, glossitis, eructation
Hepatobiliary: hepatitis, liver failure
Hemic and Lymphatic: rectal bleeding, lymphadenopathy, pancytopenia
Metabolic and Nutritional: weight changes
Nervous System: anxiety, asthenia, confusion, nervousness, paresthesia, somnolence, tremors, convulsions, coma, hallucinations
Respiratory: asthma, respiratory depression, pneumonia
Dermatologic: exfoliative dermatitis
Special Senses: blurred vision, conjunctivitis
Urogenital: cystitis, dysuria, oliguria/polyuria, proteinuria
OVERDOSAGE
Symptoms and Signs
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare. Because naproxen sodium may be rapidly absorbed, high and early blood levels should be anticipated. A few patients have experienced convulsions, but it is not clear whether or not these were drug-related. It is not known what dose of the drug would be life threatening. (see WARNINGS; Cardiovascular Thrombotic Events, Gastrointestinal Bleeding, Ulceration, and Perforation, Hypertension, Renal Toxicity and Hyperkalemia).
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Hemodialysis does not decrease the plasma concentration of naproxen because of the high degree of its protein binding. Consider emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS and other treatment options before deciding to use NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS; Gastrointestinal Bleeding, Ulceration, and Perforation).
After observing the response to initial therapy with NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM or NAPROXEN SODIUM DS, the dose and frequency should be adjusted to suit an individual patient’s needs.
Different dose strengths and formulations (ie, tablets, suspension) of the drug are not necessarily bioequivalent. This difference should be taken into consideration when changing formulation.
Although NAPROXEN, EC-NAPROXEN, NAPROXEN SODIUM and NAPROXEN SODIUM DS all circulate in the plasma as naproxen, they have pharmacokinetic differences that may affect onset of action. Onset of pain relief can begin within 30 minutes in patients taking naproxen sodium and within 1 hour in patients taking naproxen. Because EC-NAPROXEN dissolves in the small intestine rather than in the stomach, the absorption of the drug is delayed compared to the other naproxen formulations (see CLINICAL PHARMACOLOGY).
The recommended strategy for initiating therapy is to choose a formulation and a starting dose likely to be effective for the patient and then adjust the dosage based on observation of benefit and/or adverse events. A lower dose should be considered in patients with renal or hepatic impairment or in elderly patients (see WARNINGS; Hepatotoxicity, and Renal Toxicity and Hyperkalemia, and PRECAUTIONS; Geriatric Use).
Geriatric Patients
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
Patients With Moderate to Severe Renal Impairment
Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance <30 mL/min) (see WARNINGS: Renal Effects).
Rheumatoid Arthritis, Osteoarthritis and Ankylosing Spondylitis
| NAPROXEN | 250 mg or 375 mg or 500 mg | twice daily twice daily twice daily |
| NAPROXEN SODIUM | 275 mg (naproxen 250 mg with 25 mg sodium) | twice daily |
| NAPROXEN SODIUM DS | 550 mg (naproxen 500 mg with 50 mg sodium) | twice daily |
| EC-NAPROXEN | 375 mg or 500 mg | twice daily twice daily |
To maintain the integrity of the enteric coating, the EC-NAPROXEN tablet should not be broken, crushed or chewed during ingestion. NAPROXEN Suspension should be shaken gently before use.
During long-term administration, the dose of naproxen may be adjusted up or down depending on the clinical response of the patient. A lower daily dose may suffice for long-term administration. The morning and evening doses do not have to be equal in size and the administration of the drug more frequently than twice daily is not necessary.
In patients who tolerate lower doses well, the dose may be increased to naproxen 1500 mg/day for limited periods of up to 6 months when a higher level of anti-inflammatory/analgesic activity is required. When treating such patients with naproxen 1500 mg/day, the physician should observe sufficient increased clinical benefits to offset the potential increased risk. The morning and evening doses do not have to be equal in size and administration of the drug more frequently than twice daily does not generally make a difference in response (see CLINICAL PHARMACOLOGY).
Juvenile Arthritis
Naproxen Tablets may not allow for the flexible dose titration needed in pediatric patients with juvenile arthritis. A liquid formulation may be more appropriate.
In pediatric patients, doses of 5 mg/kg/day produced plasma levels of naproxen similar to those seen in adults taking 500 mg of naproxen (see CLINICAL PHARMACOLOGY).
The recommended total daily dose of naproxen is approximately 10 mg/kg given in 2 divided doses. One-half of the 250 mg tablet will be needed for dosing lower-weight children. Dosing with Naproxen Tablets is not appropriate for children weighing less than 25 kilograms.
The recommended total daily dose of naproxen is approximately 10 mg/kg given in 2 divided doses (i.e., 5 mg/kg given twice a day). A measuring cup marked in 1/2 teaspoon and 2.5 milliliter increments is provided with the naproxen suspension. The following table may be used as a guide for dosing of naproxen suspension:
Patient’s Weight Dose Administered as
13 kg (29 lb) 62.5 mg bid 2.5 mL (1/2 tsp) twice daily
25 kg (55 lb) 125 mg bid 5.0 mL (1 tsp) twice daily
38 kg (84 lb) 187.5 mg bid 7.5 mL (1 1/2 tsp) twice daily
Management of Pain, Primary Dysmenorrhea, and Acute Tendonitis and Bursitis
The recommended starting dose is 550 mg of naproxen sodium as ANAPROX/ANAPROX DS followed by 550 mg every 12 hours or 275 mg every 6 to 8 hours as required. The initial total daily dose should not exceed 1375 mg of naproxen sodium. Thereafter, the total daily dose should not exceed 1100 mg of naproxen sodium. Because the sodium salt of naproxen is more rapidly absorbed, ANAPROX/ANAPROX DS is recommended for the management of acute painful conditions when prompt onset of pain relief is desired. NAPROXEN may also be used but EC-NAPROXEN is not recommended for initial treatment of acute pain because absorption of naproxen is delayed compared to other naproxen-containing products (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE).
Acute Gout
The recommended starting dose is 750 mg of NAPROXEN followed by 250 mg every 8 hours until the attack has subsided. ANAPROX may also be used at a starting dose of 825 mg followed by 275 mg every 8 hours. EC-NAPROXEN is not recommended because of the delay in absorption (see CLINICAL PHARMACOLOGY).
HOW SUPPLIED
NAPROXEN Tablets: 250 mg: round, yellow, biconvex, engraved with NPR LE 250 on one side and scored on the other. Packaged in light-resistant bottles of 500.
500’s (bottle): NDC 42494-403-05.
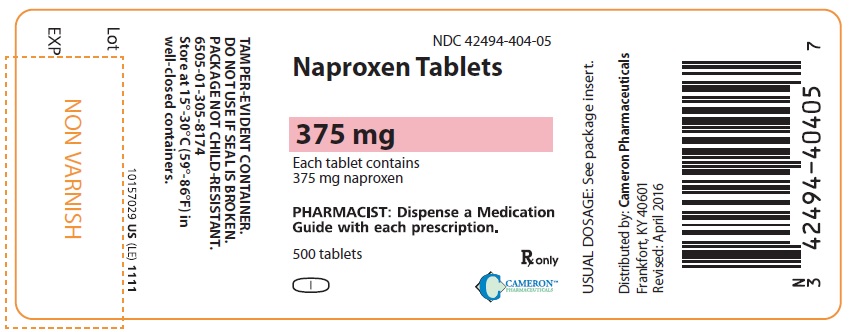
375 mg: pink, biconvex oval, engraved with NPR LE 375 on one side. Packaged in light-resistant bottles of 500.
500’s (bottle): NDC 42494-404-05.
500 mg: yellow, capsule-shaped, engraved with NPR LE 500 on one side and scored on the other. Packaged in light-resistant bottles of 100 and 1000.
100’s (bottle): NDC 42494-405-01.
1000’s (bottle): NDC 42494-406-10.
Store at 15° to 30°C (59° to 86°F) in well-closed containers; dispense in light-resistant containers.
EC-NAPROXEN Delayed-Release Tablets: 375 mg: white, oval biconvex coated tablets imprinted with NPR EC 375 on one side. Packaged in light-resistant bottles of 100.
100’s (bottle): NDC 42494-407-01.
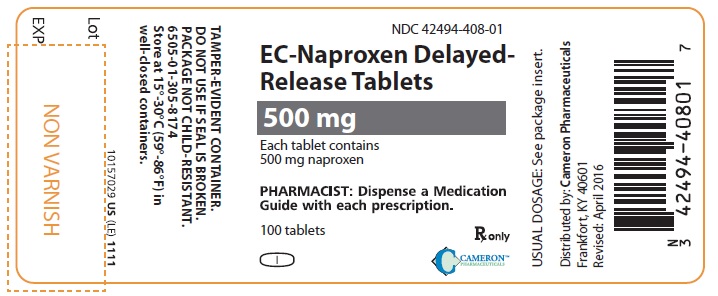
500 mg: white, oblong coated tablets imprinted with NPR EC 500 on one side. Packaged in light-resistant bottles of 100.
100’s (bottle): NDC 42494-408-01.
Store at 15° to 30°C (59° to 86°F) in well-closed containers; dispense in light-resistant containers.
NAPROXEN SODIUM Tablets:Naproxen sodium 275 mg: light blue, oval-shaped, engraved with NPS-275 on one side. Packaged in bottles of 100.
100’s (bottle): NDC 42494-402-01.
Store at 15° to 30°C (59° to 86°F) in well-closed containers.
NAPROXEN SODIUM DS Tablets:Naproxen sodium 550 mg: dark blue, oblong-shaped, engraved with NPS 550 on one side and scored on both sides. Packaged in bottles of 100 and 500.
100’s (bottle): NDC 42494-400-01.
500’s (bottle): NDC 42494-400-05.
Store at 15° to 30°C (59° to 86°F) in well-closed containers.
Manufactured for:
Cameron Pharmaceuticals, LLC
1009 Twilight Trail, Suite 107
Frankfort, Kentucky 40601
Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
-
Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
-
Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- taking medicines called "corticosteroids", "anticoagulants", "SSRIs", or "SNRIs"
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
NSAIDs should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.
Who should not take NSAIDs?
Do not take NSAIDs:
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
- right before or after heart bypass surgery.
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have high blood pressure
- have asthma
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you are considering taking NSAIDs during pregnancy. You should not take NSAIDs after 29 weeks of pregnancy
- are breastfeeding or plan to breast feed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. NSAIDs and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.
What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See "What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
- new or worse high blood pressure
- heart failure
- liver problems including liver failure
- kidney problems including kidney failure
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
- Other side effects of NSAIDs include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
Get emergency help right away if you get any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands and feet
If you take too much of your NSAID, call your healthcare provider or get medical help right away.
These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
Manufactured for:
Cameron Pharmaceuticals, LLC
1009 Twilight Trail, Suite 107
Frankfort, Kentucky 40601
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: May 2016
All registered trademarks in this document are the property of their respective owners.
Revised: July 2016
PRINCIPAL DISPLAY PANEL
NDC 42494-407-01
EC- Naproxen Delayed- Release Tablets
375 mg
100 Tablets
Rx Only