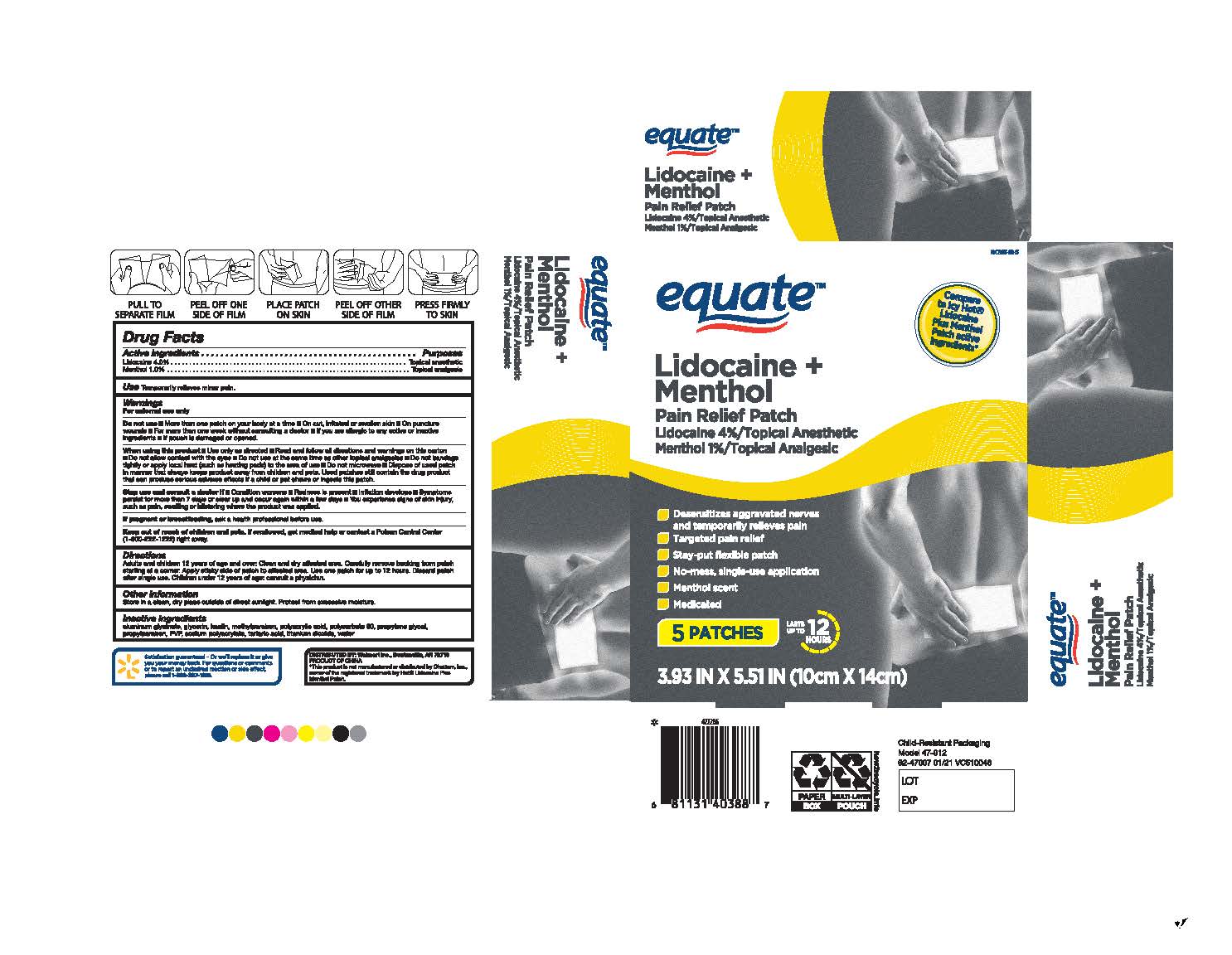
- Warnings
For external use only.
Use only as directed
Read and follow all directions and warnings on this carton
Do not allow contact with the eyes
Do not use at the same time as other topical analgesics
Do not bandage tightly or apply local heat (such as heating pads) to the area of use
Do not microwave
Dispose of the used patch in a manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
More than one patch on your body at a time.
On cut, irritated or swollen skin
On puncture wounds
For more than one week without consulting a doctor
If you are allergic to any active or inactive ingredients
If pouch is damaged or opened
Directions
Adults and children 12 years of age and over: Clean and the dry affected area. Carefully remove backing from the patch starting at a corner. Apply the sticky side of patch to the affected area. Use one patch for up to 12 hours. Discard patch after a single use. Children under 12 years old of age: consult a physician.