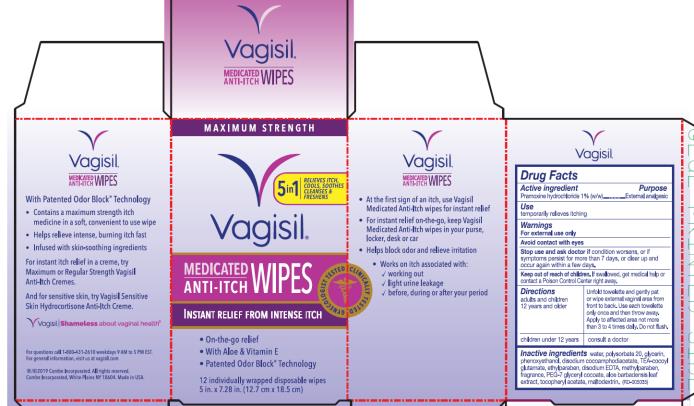
Stop use and ask doctor if
condition worsens, or if symptoms persist for more than 7 days, or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years and older | Unfold towelette and gently pat or wipe external vaginal area from front to back. Use each towelette only once and then throw away. Apply to affected area not more than 3 to 4 times daily. Do not flush. |
| children under 12 years | consult a doctor |
Inactive ingredients
water, polysorbate 20, glycerin, phenoxyethanol, disodium cocoamphodiacetate, TEA-cocoyl glutamate, ethylparaben, disodium EDTA, methylparaben, fragrance, PEG-7 glyceryl cocoate, aloe barbadensis leaf extract, tocopheryl acetate, maltodextrin
Stop use and ask doctor if
condition worsens, or if symptoms persist for more than 7 days, or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years and older | Unfold towelette and gently pat or wipe external vaginal area from front to back. Use each towelette only once and then throw away. Apply to affected area not more than 3 to 4 times daily. Do not flush. |
| children under 12 years | consult a doctor |
Inactive ingredients
water, polysorbate 20, glycerin, phenoxyethanol, disodium cocoamphodiacetate, TEA-cocoyl glutamate, ethylparaben, disodium EDTA, methylparaben, fragrance, PEG-7 glyceryl cocoate, aloe barbadensis leaf extract, tocopheryl acetate, maltodextrin