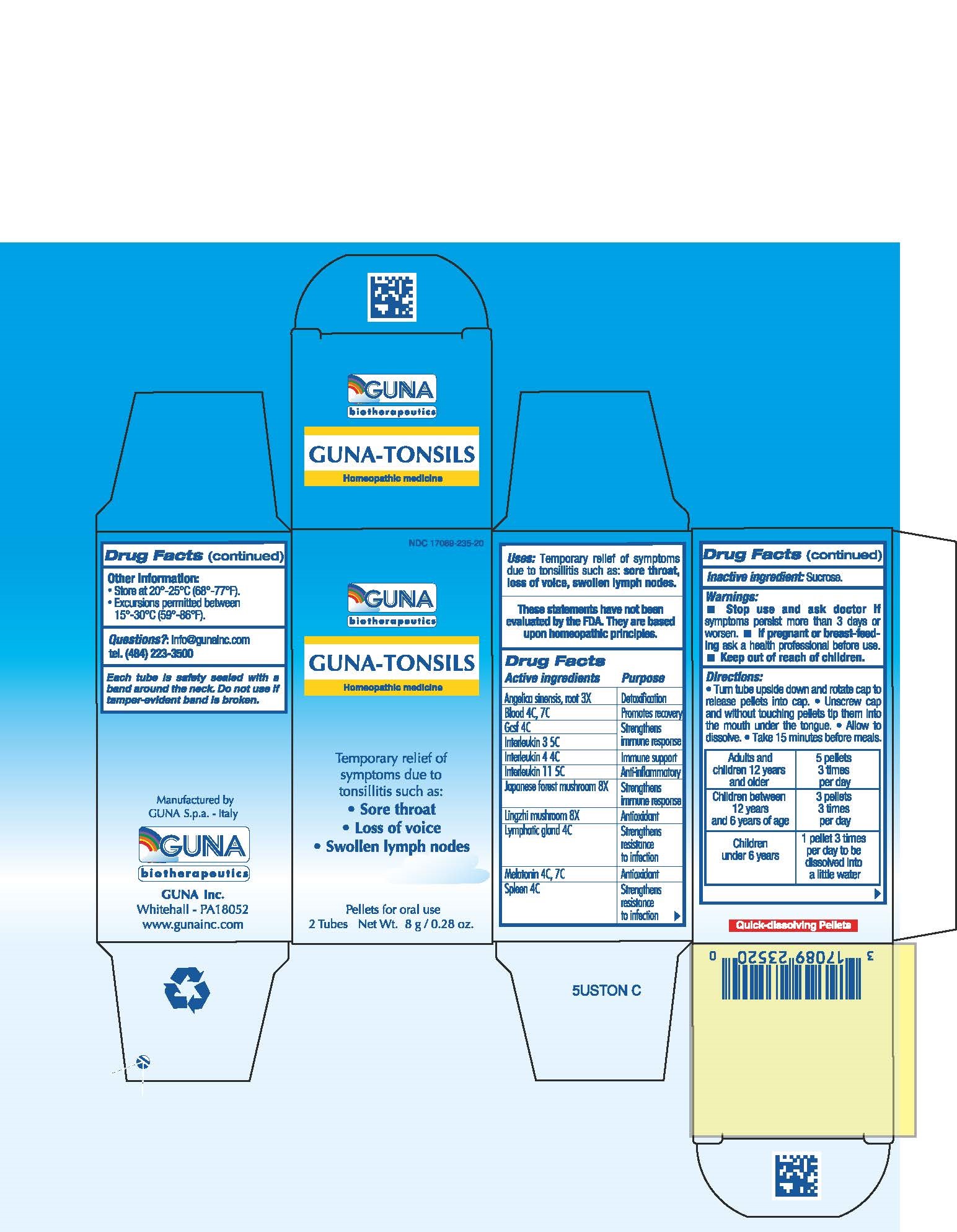
ACTIVE INGREDIENTS/PURPOSE
ANGELICA SINENSIS, RADIX 3X DETOXIFICATION
BLOOD 4C, 7C PROMOTES RECOVERY
GCSF 4C STRENGTHENS IMMUNE RESPONSE
INTERLEUKIN 11 5C ANTI-INFLAMMATORY
INTERLEUKIN 3 5C STRENGTHENS IMMUNE RESPONSE
INTERLEUKIN 4 4C IMMUNE SUPPORT
JAPANESE FOREST MUSHROOM 8X STRENGTHENS IMMUNE RESPONSE
LING CHI MUSHROOM 8X ANTIOXIDANT
LYMPHATIC GLAND 4C STRENGTHENS RESISTANCE TO INFECTION
MELATONIN 4C, 7C ANTIOXIDANT
SPLEEN 4C STRENGTHENS RESISTANCE TO INFECTION
USES
Temporary relief of symptoms due to Tonsillitis such as:
- Sore throat
- Loss of voice
-
Swollen lymph nodes
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days or worsen.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children.
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 5 pellets 3 times per day
Children between 12 years and 6 years of age 3 pellets 3 times per day
Children under 6 years 1 pellet 3 times per day to be dissolved into a little water