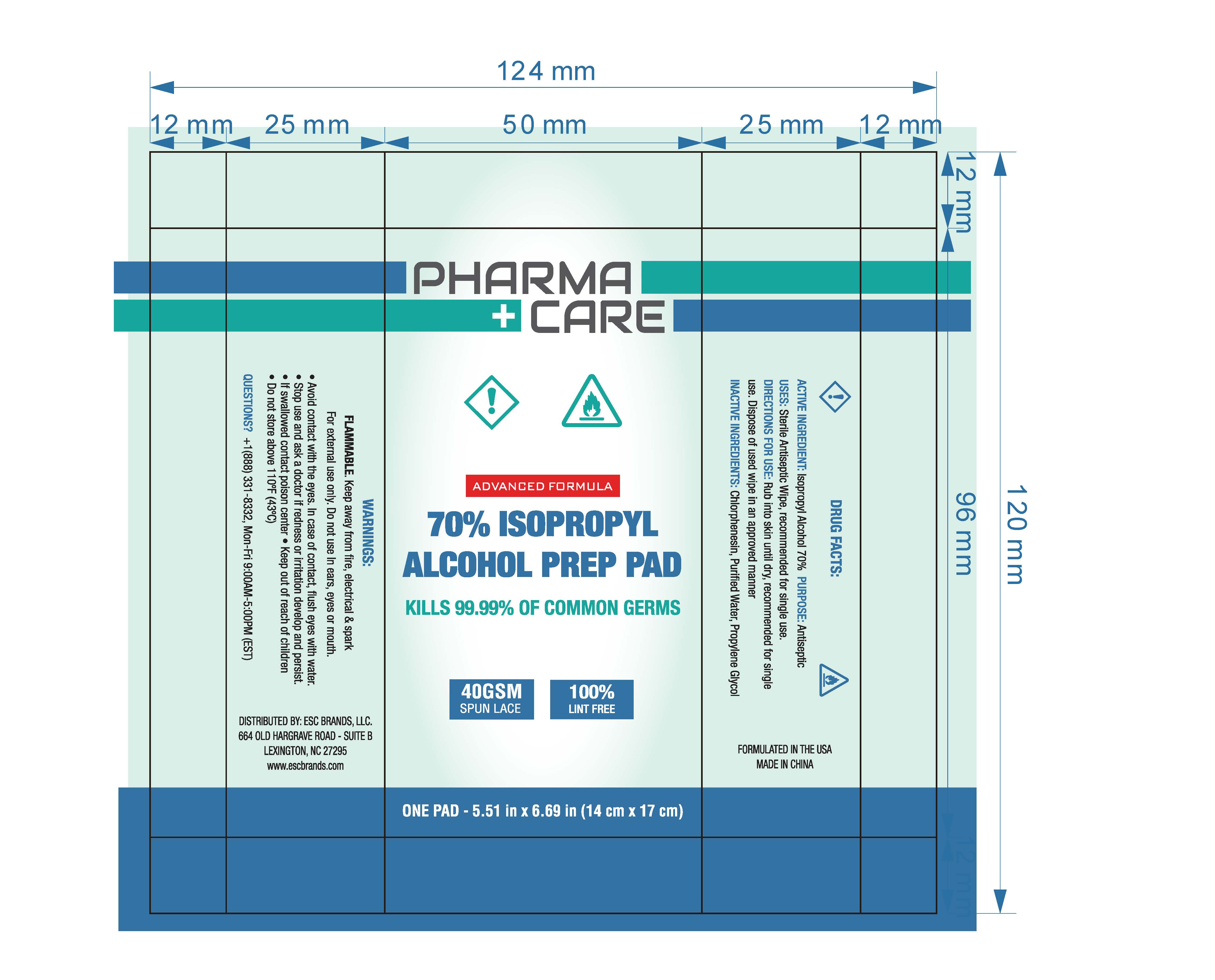
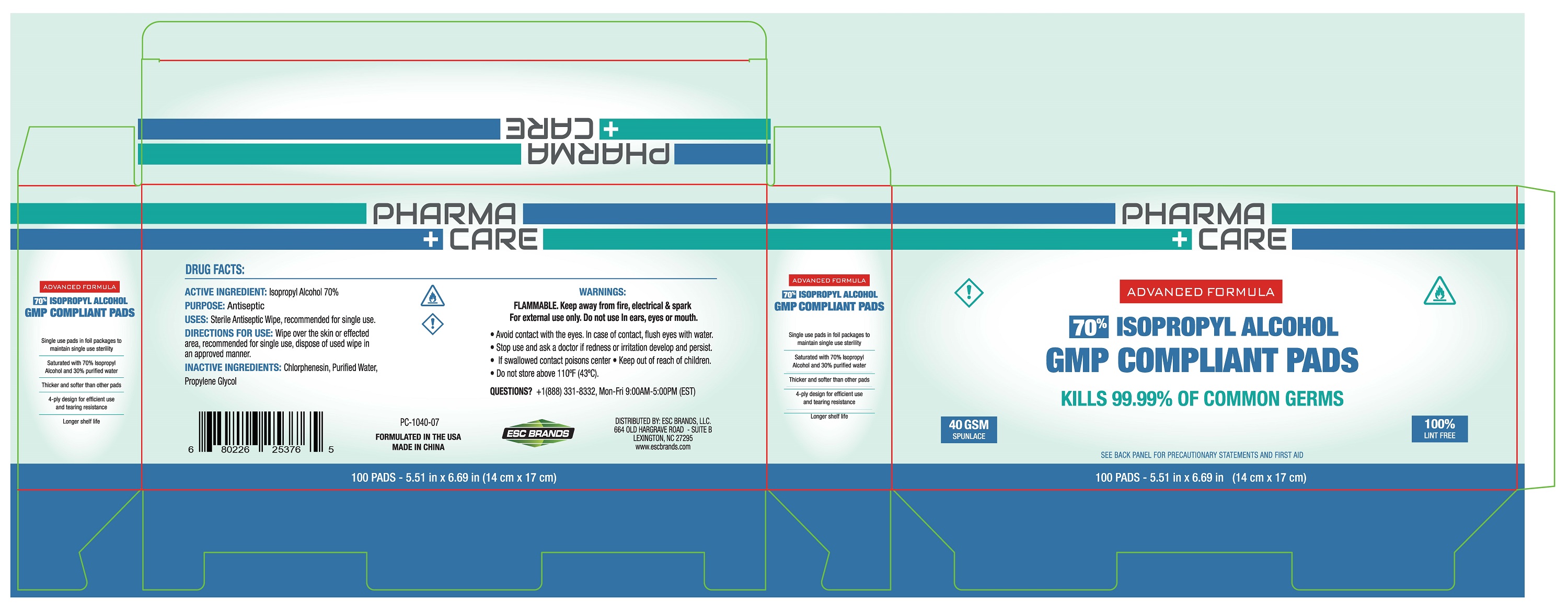
70% ISOPROPYL ALCOHOL PREP PAD- isopropyl alcohol cloth
Landy International
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient(s)
ACTIVE INGREDIENT: Isopropyl Alcohol 70%
Use
USES: Sterile antiseptic wipe, recommended for single use.
Warnings
FLAMMABLE. Keep away from fire, electrical & spark
For external use only. Do not use In ears, eyes or mouth.
●Avoid contact with the eyes. In case of contact, flush eyes with water
●Stop use and ask a doctor if redness or iritation develop
●If swallowed contact a poison center
Keep out of reach of children.
Directions
Wipe over the skin or affected area, recommended for single use, dispose of used wipe in an approved manner.
Inactive ingredients
INACTIVE INGREDIENTS: Chlorphenesin, Purified Water,
Propylene Glycol