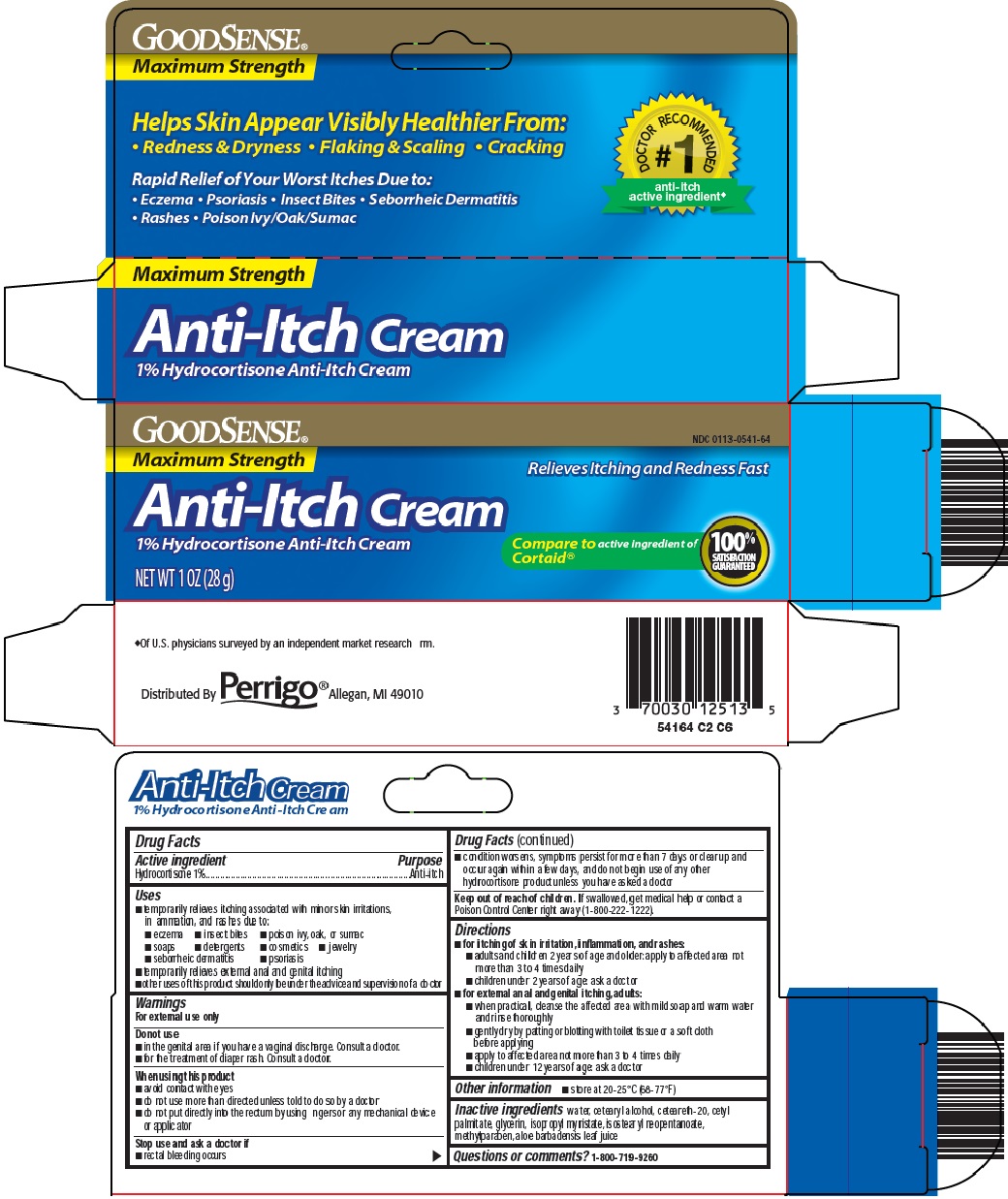
Uses
- •
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- •
- eczema
- •
- insect bites
- •
- poison ivy, oak, or sumac
- •
- soaps
- •
- detergents
- •
- cosmetics
- •
- jewelry
- •
- seborrheic dermatitis
- •
- psoriasis
- •
- temporarily relieves external anal and genital itching
- •
- other uses of this product should only be under the advice and supervision of a doctor
Warnings
For external use only
Do not use
- •
- in the genital area if you have a vaginal discharge. Consult a doctor.
- •
- for the treatment of diaper rash. Consult a doctor.
When using this product
- •
- avoid contact with eyes
- •
- do not use more than directed unless told to do so by a doctor
- •
- do not put directly into the rectum by using fingers or any mechanical device or applicator
Directions
- •
- for itching of skin irritation, inflammation, and rashes:
- •
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- •
- children under 2 years of age: ask a doctor
- •
- for external anal and genital itching, adults:
- •
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- •
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- •
- apply to affected area not more than 3 to 4 times daily
- •
- children under 12 years of age: ask a doctor
Inactive ingredients
water, cetearyl alcohol, ceteareth-20, cetyl palmitate, glycerin, isopropyl myristate, isostearyl neopentanoate, methylparaben, aloe barbadensis leaf juice
Principal Display Panel
Maximum Strength
Helps Skin Appear Visibly Healthier From:
Redness & Dryness ● Flaking & Scaling ● Cracking
#1 DOCTOR RECOMMENDED anti-itch active ingredient
Rapid Relief of Your Worst Itches Due to:
Eczema ● Psoriasis ● Insect Bites ● Seborrheic Dermatitis
Rashes ● Poison Ivy/Oak/Sumac
Maximum Strength
Relieves Itching and Redness Fast
Anti-Itch Cream
1% Hydrocortisone Anti-Itch Cream
Compare to active ingredient of Cortaid®
100% SATISFACTION GUARANTEED
NET WT 1 OZ (28 g)