DESCRIPTION
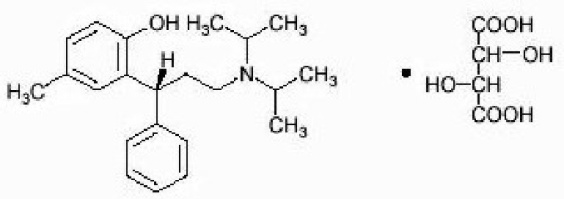
This product contains tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is (R)-2-[3-[bis(1-methylethyl)-amino]1-phenylpropyl]-4-methylphenol [R-(R*,R*)]-2,3dihydroxybutanedioate (1:1) (salt). It has the following structural formula:
C26H37NO7 M.W. 475.6
Tolterodine tartrate is a white, crystalline powder. The pKa value is 9.87 and the solubility in water is 12 mg/mL. It is soluble in methanol, slightly soluble in ethanol, and practically insoluble in toluene. The partition coefficient (Log D) between n-octanol and water is 1.83 at pH 7.3.
Each tolterodine tartrate tablet, for oral administration, contains 1 mg or 2 mg of tolterodine tartrate. In addition, each tablet contains the following inactive ingredients: corn starch, croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, sodium stearyl fumarate, and titanium dioxide.
CLINICAL PHARMACOLOGY
Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors.
After oral administration, tolterodine is metabolized in the liver, resulting in the formation of the 5-hydroxymethyl derivative, a major pharmacologically active metabolite. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and the 5-hydroxymethyl metabolite exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels.
Tolterodine has a pronounced effect on bladder function. Effects on urodynamic parameters before and 1 and 5 hours after a single 6.4 mg dose of tolterodine immediate-release were determined in healthy volunteers. The main effects of tolterodine at 1 and 5 hours were an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure. These findings are consistent with an antimuscarinic action on the lower urinary tract.
Pharmacokinetics
Absorption
In a study with 14C-tolterodine solution in healthy volunteers who received a 5 mg oral dose, at least 77% of the radiolabeled dose was absorbed. Tolterodine immediate-release is rapidly absorbed, and maximum serum concentrations (Cmax) typically occur within 1 to 2 hours after dose administration. Cmax and area under the concentration-time curve (AUC) determined after dosage of tolterodine immediate-release are dose-proportional over the range of 1 to 4 mg.
Effect of Food
Food intake increases the bioavailability of tolterodine (average increase 53%), but does not affect the levels of the 5-hydroxymethyl metabolite in extensive metabolizers. This change is not expected to be a safety concern and adjustment of dose is not needed.
Distribution
Tolterodine is highly bound to plasma proteins, primarily α1-acid glycoprotein. Unbound concentrations of tolterodine average 3.7% ± 0.13% over the concentration range achieved in clinical studies. The 5-hydroxymethyl metabolite is not extensively protein bound, with unbound fraction concentrations averaging 36% ± 4%. The blood to serum ratio of tolterodine and the 5-hydroxymethyl metabolite averages 0.6 and 0.8, respectively, indicating that these compounds do not distribute extensively into erythrocytes. The volume of distribution of tolterodine following administration of a 1.28 mg intravenous dose is 113 ± 26.7 L.
Metabolism
Tolterodine is extensively metabolized by the liver following oral dosing. The primary metabolic route involves the oxidation of the 5-methyl group and is mediated by the cytochrome P450 2D6 (CYP2D6) and leads to the formation of a pharmacologically active 5-hydroxymethyl metabolite. Further metabolism leads to formation of the 5-carboxylic acid and N-dealkylated 5-carboxylic acid metabolites, which account for 51% ± 14% and 29% ± 6.3% of the metabolites recovered in the urine, respectively.
Variability in Metabolism
A subset (about 7%) of the population is devoid of CYP2D6, the enzyme responsible for the formation of the 5-hydroxymethyl metabolite of tolterodine. The identified pathway of metabolism for these individuals ("poor metabolizers") is dealkylation via cytochrome P450 3A4 (CYP3A4) to N-dealkylated tolterodine. The remainder of the population is referred to as "extensive metabolizers." Pharmacokinetic studies revealed that tolterodine is metabolized at a slower rate in poor metabolizers than in extensive metabolizers; this results in significantly higher serum concentrations of tolterodine and in negligible concentrations of the 5-hydroxymethyl metabolite.
Excretion
Following administration of a 5 mg oral dose of 14C-tolterodine solution to healthy volunteers, 77% of radioactivity was recovered in urine and 17% was recovered in feces in 7 days. Less than 1% (< 2.5% in poor metabolizers) of the dose was recovered as intact tolterodine, and 5% to 14% (< 1% in poor metabolizers) was recovered as the active 5-hydroxymethyl metabolite.
A summary of mean (± standard deviation) pharmacokinetic parameters of tolterodine immediate-release and the 5-hydroxymethyl metabolite in extensive (EM) and poor (PM) metabolizers is provided in Table 1. These data were obtained following single- and multiple-doses of tolterodine 4 mg administered twice daily to 16 healthy male volunteers (8 EM, 8 PM).
|
|
Tolterodine |
5-Hydroxymethyl Metabolite |
|||||||
|
Phenotype(CYP2D6) |
tmax(h) |
Cmax*(mcg/L) |
Cavg*(mcg/L) |
t1/2(h) |
CL/F(L/h) |
tmax(h) |
Cmax*(mcg/L) |
Cavg*(mcg/L) |
t1/2(h) |
|
Single-dose | |||||||||
|
EM |
1.6±1.5 |
1.6±1.2 |
0.50±0.35 |
2±0.7 |
534±697 |
1.8±1.4 |
1.8±0.7 |
0.62±0.26 |
3.1±0.7 |
|
PM |
1.4±0.5 |
10±4.9 |
8.3±4.3 |
6.5±1.6 |
17±7.3 | ||||
|
Multiple-dose | |||||||||
|
EM |
1.2±0.5 |
2.6±2.8 |
0.58±0.54 |
2.2±0.4 |
415±377 |
1.2±0.5 |
2.4±1.3 |
0.92±0.46 |
2.9±0.4 |
|
PM |
1.9±1 |
19±7.5 |
12±5.1 |
9.6±1.5 |
11±4.2 | ||||
Cmax = Maximum plasma concentration; tmax = Time of occurrence of Cmax;
Cavg = Average plasma concentration; t1/2 = Terminal elimination half-life; CL/F = Apparent oral clearance.
EM = Extensive metabolizers; PM = Poor metabolizers.
Pharmacokinetics in Special Populations
Age
In Phase 1, multiple-dose studies in which tolterodine immediate-release 4 mg (2 mg BID) was administered, serum concentrations of tolterodine and of the 5-hydroxymethyl metabolite were similar in healthy elderly volunteers (aged 64 through 80 years) and healthy young volunteers (aged less than 40 years). In another Phase 1 study, elderly volunteers (aged 71 through 81 years) were given tolterodine immediate-release 2 or 4 mg (1 or 2 mg BID). Mean serum concentrations of tolterodine and the 5-hydroxymethyl metabolite in these elderly volunteers were approximately 20% and 50% higher, respectively, than reported in young healthy volunteers. However, no overall differences were observed in safety between older and younger patients on tolterodine in Phase 3, 12 week, controlled clinical studies; therefore, no tolterodine dosage adjustment for elderly patients is recommended (see PRECAUTIONS, Geriatric Use).
Gender
The pharmacokinetics of tolterodine immediate-release and the 5-hydroxymethyl metabolite are not influenced by gender. Mean Cmax of tolterodine (1.6 mcg/L in males versus 2.2 mcg/L in females) and the active 5-hydroxymethyl metabolite (2.2 mcg/L in males versus 2.5 mcg/L in females) are similar in males and females who were administered tolterodine immediate-release 2 mg. Mean AUC values of tolterodine (6.7 mcg•h/L in males versus 7.8 mcg•h/L in females) and the 5-hydroxymethyl metabolite (10 mcg•h/L in males versus 11 mcg•h/L in females) are also similar. The elimination half-life of tolterodine for both males and females is 2.4 hours, and the half-life of the 5-hydroxymethyl metabolite is 3 hours in females and 3.3 hours in males.
Renal Insufficiency
Renal impairment can significantly alter the disposition of tolterodine immediate-release and its metabolites. In a study conducted in patients with creatinine clearance between 10 and 30 mL/min, tolterodine immediate-release and the 5-hydroxymethyl metabolite levels were approximately 2 to 3 fold higher in patients with renal impairment than in healthy volunteers. Exposure levels of other metabolites of tolterodine (e.g., tolterodine acid, N-dealkylated tolterodine acid, N-dealkylated tolterodine, and N-dealkylated hydroxylated tolterodine) were significantly higher (10 to 30 fold) in renally impaired patients as compared to the healthy volunteers. The recommended dosage for patients with significantly reduced renal function is tolterodine 1 mg twice daily (see PRECAUTIONS, General and DOSAGE AND ADMINISTRATION).
Hepatic Insufficiency
Liver impairment can significantly alter the disposition of tolterodine immediate-release. In a study conducted in cirrhotic patients, the elimination half-life of tolterodine immediate-release was longer in cirrhotic patients (mean, 7.8 hours) than in healthy, young, and elderly volunteers (mean, 2 to 4 hours). The clearance of orally administered tolterodine was substantially lower in cirrhotic patients (1 ± 1.7 L/h/kg) than in the healthy volunteers (5.7 ± 3.8 L/h/kg). The recommended dose for patients with significantly reduced hepatic function is tolterodine 1 mg twice daily (see PRECAUTIONS, General and DOSAGE AND ADMINISTRATION).
Drug-Drug Interactions
Fluoxetine
Fluoxetine is a selective serotonin reuptake inhibitor and a potent inhibitor of CYP2D6 activity. In a study to assess the effect of fluoxetine on the pharmacokinetics of tolterodine immediate-release and its metabolites, it was observed that fluoxetine significantly inhibited the metabolism of tolterodine immediate-release in extensive metabolizers, resulting in a 4.8 fold increase in tolterodine AUC. There was a 52% decrease in Cmax and a 20% decrease in AUC of the 5-hydroxymethyl metabolite. Fluoxetine thus alters the pharmacokinetics in patients who would otherwise be extensive metabolizers of tolterodine immediate-release to resemble the pharmacokinetic profile in poor metabolizers. The sums of unbound serum concentrations of tolterodine immediate-release and the 5-hydroxymethyl metabolite are only 25% higher during the interaction. No dose adjustment is required when tolterodine and fluoxetine are coadministered.
Other Drugs Metabolized by Cytochrome P450 Isoenzymes
Tolterodine immediate-release does not cause clinically significant interactions with other drugs metabolized by the major drug metabolizing CYP enzymes. In vivo drug interaction data show that tolterodine immediate-release does not result in clinically relevant inhibition of CYP1A2, 2D6, 2C9, 2C19, or 3A4 as evidenced by lack of influence on the marker drugs caffeine, debrisoquine, S-warfarin, and omeprazole. In vitro data show that tolterodine immediate-release is a competitive inhibitor of CYP2D6 at high concentrations (Ki 1.05 µM), while tolterodine immediate-release as well as the 5-hydroxymethyl metabolite are devoid of any significant inhibitory potential regarding the other isoenzymes.
CYP3A4 Inhibitors
The effect of 200 mg daily dose of ketoconazole on the pharmacokinetics of tolterodine immediate-release was studied in 8 healthy volunteers, all of whom were poor metabolizers (see Pharmacokinetics, Variability in Metabolism for discussion of poor metabolizers). In the presence of ketoconazole, the mean Cmax and AUC of tolterodine increased by 2 and 2.5 fold, respectively. Based on these findings, other potent CYP3A inhibitors such as other azole antifungals (e.g., itraconazole, miconazole) or macrolide antibiotics (e.g., erythromycin, clarithromycin) or cyclosporine or vinblastine may also lead to increases of tolterodine plasma concentrations (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Warfarin
In healthy volunteers, coadministration of tolterodine immediate-release 4 mg (2 mg BID) for 7 days and a single dose of warfarin 25 mg on day 4 had no effect on prothrombin time, Factor VII suppression, or on the pharmacokinetics of warfarin.
Oral Contraceptives
Tolterodine immediate-release 4 mg (2 mg BID) had no effect on the pharmacokinetics of an oral contraceptive (ethinyl estradiol 30 mcg/levonorgestrel 150 mcg) as evidenced by the monitoring of ethinyl estradiol and levonorgestrel over a 2 month cycle in healthy female volunteers.
Diuretics
Coadministration of tolterodine immediate-release up to 8 mg (4 mg BID) for up to 12 weeks with diuretic agents, such as indapamide, hydrochlorothiazide, triamterene, bendroflumethiazide, chlorothiazide, methylchlorothiazide, or furosemide, did not cause any adverse electrocardiographic (ECG) effects.
Cardiac Electrophysiology
The effect of 2 mg BID and 4 mg BID of tolterodine immediate-release (IR) on the QT interval was evaluated in a 4 way crossover, double-blind, placebo- and active-controlled (moxifloxacin 400 mg QD) study in healthy male (N = 25) and female (N = 23) volunteers aged 18 to 55 years. Study subjects [approximately equal representation of CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs)] completed sequential 4 day periods of dosing with moxifloxacin 400 mg QD, tolterodine 2 mg BID, tolterodine 4 mg BID, and placebo. The 4 mg BID dose of tolterodine IR (two times the highest recommended dose) was chosen because this dose results in tolterodine exposure similar to that observed upon coadministration of tolterodine 2 mg BID with potent CYP3A4 inhibitors in patients who are CYP2D6 poor metabolizers (see PRECAUTIONS, Drug Interactions). QT interval was measured over a 12 hour period following dosing, including the time of peak plasma concentration (Tmax) of tolterodine and at steady state (Day 4 of dosing).
Table 2 summarizes the mean change from baseline to steady state in corrected QT interval (QTc) relative to placebo at the time of peak tolterodine (1 hour) and moxifloxacin (2 hour) concentrations. Both Fridericia’s (QTcF) and a population-specific (QTcP) method were used to correct QT interval for heart rate. No single QT correction method is known to be more valid than others. QT interval was measured manually and by machine, and data from both are presented. The mean increase of heart rate associated with a 4 mg/day dose of tolterodine in this study was 2 beats/minute and 6.3 beats/minute with 8 mg/day tolterodine. The change in heart rate with moxifloxacin was 0.5 beats/minute
Table 2: Mean (CI) Change in QTc From Baseline to Steady State
|
Drug/Dose |
N |
QTcF (msec) (manual) |
QTcF (msec) (machine) |
QTcP (msec) (manual) |
QTcP (msec) (machine) |
|
Tolterodine 2 mg BID* |
48 |
5.01 (0.28, 9.74) |
1.16 (-2.99, 5.30) |
4.45 (-0.37, 9.26) |
2 (-1.81, 5.81) |
|
Tolterodine 4 mg BID* |
48 |
11.84 (7.11, 16.58) |
5.63 (1.48, 9.77) |
10.31 (5.49, 15.12) |
8.34 (4.53, 12.15) |
|
Moxifloxacin 400 mg QD† |
45 |
19.26‡ (15.49, 23.03) |
8.90 (4.77, 13.03) |
19.10‡ (15.32, 22.89) |
9.29 (5.34, 13.24) |
The reason for the difference between machine and manual read of QT interval is unclear.
The QT effect of tolterodine immediate-release tablets appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day. The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped.
Tolterodine’s effect on QT interval was found to correlate with plasma concentration of tolterodine. There appeared to be a greater QTc interval increase in CYP2D6 poor metabolizers than in CYP2D6 extensive metabolizers after tolterodine treatment in this study.
This study was not designed to make direct statistical comparisons between drugs or dose levels. There has been no association of Torsade de pointes in the international postmarketing experience with tolterodine tablets or tolterodine extended-release capsules (see PRECAUTIONS, Patients with Congenital or Acquired QT Prolongation).
CLINICAL STUDIES
Tolterodine tablets were evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in four randomized, double-blind, placebo-controlled, 12 week studies. A total of 853 patients received tolterodine 2 mg twice daily and 685 patients received placebo. The majority of patients were Caucasian (95%) and female (78%), with a mean age of 60 years (range, 19 to 93 years). At study entry, nearly all patients perceived they had urgency and most patients had increased frequency of micturitions and urge incontinence. These characteristics were well balanced across treatment groups for the studies.
The efficacy endpoints for study 007 (see Table 3) included the change from baseline for:
- •
- Number of incontinence episodes per week
- •
- Number of micturitions per 24 hours (averaged over 7 days)
- •
- Volume of urine voided per micturition (averaged over 2 days)
The efficacy endpoints for studies 008, 009, and 010 (see Table 4) were identical to the above endpoints with the exception that the number of incontinence episodes was per 24 hours (averaged over 7 days).
|
|||
|
Tolterodine(SD) N = 514 |
Placebo(SD) N = 508 |
Difference(95% CI) |
|
|
Number of Incontinence Episodes per Week | |||
|
Mean baseline |
23.2 |
23.3 | |
|
Mean change from baseline |
-10.6 (17) |
-6.9 (15) |
-3.7 (-5.7, -1.6) |
|
Number of Micturitions per 24 Hours | |||
|
Mean baseline |
11.1 |
11.3 | |
|
Mean change from baseline |
-1.7 (3.3) |
-1.2 (2.9) |
-0.5* (-0.9, -0.1) |
|
Volume Voided per Micturition (mL) | |||
|
Mean baseline |
137 |
136 | |
|
Mean change from baseline |
29 (47) |
14 (41) |
15* (9, 21) |
SD = Standard Deviation
|
||||
|
Study |
|
Tolterodine(SD) |
Placebo(SD) |
Difference (95% CI) |
|
Number of Incontinence Episodes per 24 Hours |
||||
|
008 |
Number of patients |
93 |
40 | |
|
Mean baseline |
2.9 |
3.3 | ||
|
Mean change from baseline |
-1.3 (3.2) |
-0.9 (1.5) |
0.5 (-1.3, 0.3) |
|
|
009 |
Number of patients |
116 |
55 | |
|
Mean baseline |
3.6 |
3.5 | ||
|
Mean change from baseline |
-1.7 (2.5) |
-1.3 (2.5) |
-0.4 (-1, 0.2) |
|
|
010 |
Number of patients |
90 |
50 | |
|
Mean baseline |
3.7 |
3.5 | ||
|
Mean change from baseline |
-1.6 (2.4) |
-1.1 (2.1) |
-0.5 (-1.1, 0.1) |
|
|
Number of Micturitions per 24 Hours |
||||
|
008 |
Number of patients |
118 |
56 | |
|
Mean baseline |
11.5 |
11.7 | ||
|
Mean change from baseline |
-2.7 (3.8) |
-1.6 (3.6) |
-1.2* (-2, -0.4) |
|
|
009 |
Number of patients |
128 |
64 | |
|
Mean baseline |
11.2 |
11.3 | ||
|
Mean change from baseline |
-2.3 (2.1) |
-1.4 (2.8) |
-0.9* (-1.5, -0.3) |
|
|
010 |
Number of patients |
108 |
56 | |
|
Mean baseline |
11.6 |
11.6 | ||
|
Mean change from baseline |
-1.7 (2.3) |
-1.4 (2.8) |
-0.38 (-1.1, 0.3) |
|
|
Volume Voided per Micturition (mL) |
||||
|
008 |
Number of patients |
118 |
56 | |
|
Mean baseline |
166 |
157 | ||
|
Mean change from baseline |
38 (54) |
6 (42) |
32* (18, 46) |
|
|
009 |
Number of patients |
129 |
64 | |
|
Mean baseline |
155 |
158 | ||
|
Mean change from baseline |
36 (50) |
10 (47) |
26* (14, 38) |
|
|
010 |
Number of patients |
108 |
56 | |
|
Mean baseline |
155 |
160 | ||
|
Mean change from baseline |
31 (45) |
13 (52) |
18* (4, 32) |
|
SD = Standard Deviation
INDICATIONS AND USAGE
Tolterodine tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
CONTRAINDICATIONS
Tolterodine tablets are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. Tolterodine tablets are also contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like tolterodine tablets, are metabolized to 5-hydroxymethyl tolterodine.
WARNINGS
Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of tolterodine tablets. In the event of difficulty in breathing, upper airway obstruction, or fall in blood pressure, tolterodine tablets should be discontinued and appropriate therapy promptly provided.
PRECAUTIONS
General
Risk of Urinary Retention and Gastric Retention
Tolterodine tablets should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention and to patients with gastrointestinal obstructive disorders, such as pyloric stenosis, because of the risk of gastric retention (see CONTRAINDICATIONS).
Decreased Gastrointestinal Motility
Tolterodine, like other antimuscarinic drugs, should be used with caution in patients with decreased gastrointestinal motility.
Controlled Narrow-Angle Glaucoma
Tolterodine should be used with caution in patients being treated for narrow-angle glaucoma.
Central Nervous System (CNS) Effects
Tolterodine tablets are associated with anticholinergic central nervous system (CNS) effects including dizziness and somnolence (see ADVERSE REACTIONS). Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until the drug’s effects have been determined. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
Reduced Hepatic and Renal Function
For patients with significantly reduced hepatic function or renal function, the recommended dose of tolterodine is 1 mg twice daily (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations).
Patients With Congenital or Acquired QT Prolongation
In a study of the effect of tolterodine immediate-release tablets on the QT interval (see CLINICAL PHARMACOLOGY, Cardiac Electrophysiology), the effect on the QT interval appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day and was more pronounced in CYP2D6 poor metabolizers (PMs) than extensive metabolizers (EMs). The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped. These observations should be considered in clinical decisions to prescribe tolterodine for patients with a known history of QT prolongation or patients who are taking Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications (see PRECAUTIONS, Drug Interactions). There has been no association of Torsade de pointes in the international postmarketing experience with tolterodine tablets or tolterodine extended-release capsules.
Information for Patients
Patients should be informed that antimuscarinic agents such as tolterodine may produce the following effects: blurred vision, dizziness, or drowsiness. Patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug's effects have been determined.
Drug Interactions
CYP3A4 Inhibitors
Ketoconazole, an inhibitor of the drug-metabolizing enzyme CYP3A4, significantly increased plasma concentrations of tolterodine when coadministered to subjects who were poor metabolizers (see CLINICAL PHARMACOLOGY, Variability in Metabolism and Drug-Drug Interactions). For patients receiving ketoconazole or other potent CYP3A4 inhibitors such as other azole antifungals (e.g., itraconazole, miconazole) or macrolide antibiotics (e.g., erythromycin, clarithromycin) or cyclosporine or vinblastine, the recommended dose of tolterodine is 1 mg twice daily (see DOSAGE AND ADMINISTRATION).
Drug-Laboratory Test Interactions
Interactions between tolterodine and laboratory tests have not been studied.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with tolterodine were conducted in mice and rats. At the maximum tolerated dose in mice (30 mg/kg/day), female rats (20 mg/kg/day), and male rats (30 mg/kg/day), AUC values obtained for tolterodine were 355, 291, and 462 mcg•h/L, respectively. In comparison, the human AUC value for a 2 mg dose administered twice daily is estimated at 34 mcg•h/L. Thus, tolterodine exposure in the carcinogenicity studies was 9 to 14 fold higher than expected in humans. No increase in tumors was found in either mice or rats.
No mutagenic effects of tolterodine were detected in a battery of in vitro tests, including bacterial mutation assays (Ames test) in 4 strains of Salmonella typhimurium and in 2 strains of Escherichia coli, a gene mutation assay in L5178Y mouse lymphoma cells, and chromosomal aberration tests in human lymphocytes. Tolterodine was also negative in vivo in the bone marrow micronucleus test in the mouse.
In female mice treated for 2 weeks before mating and during gestation with 20 mg/kg/day (corresponding to AUC value of about 500 mcg•h/L), neither effects on reproductive performance or fertility were seen. Based on AUC values, the systemic exposure was about 15 fold higher in animals than in humans. In male mice, a dose of 30 mg/kg/day did not induce any adverse effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
At oral doses of 20 mg/kg/day (approximately 14 times the human exposure), no anomalies or malformations were observed in mice. When given at doses of 30 to 40 mg/kg/day, tolterodine has been shown to be embryolethal, reduce fetal weight, and increase the incidence of fetal abnormalities (cleft palate, digital abnormalities, intra-abdominal hemorrhage, and various skeletal abnormalities, primarily reduced ossification) in mice. At these doses, the AUC values were about 20 to 25 fold higher than in humans. Rabbits treated subcutaneously at a dose of 0.8 mg/kg/day achieved an AUC of 100 mcg•h/L, which is about 3 fold higher than that resulting from the human dose. This dose did not result in any embryotoxicity or teratogenicity. There are no studies of tolterodine in pregnant women. Therefore, tolterodine should be used during pregnancy only if the potential benefit for the mother justifies the potential risk to the fetus.
Nursing Mothers
Tolterodine is excreted into the milk in mice. Offspring of female mice treated with tolterodine 20 mg/kg/day during the lactation period had slightly reduced body weight gain. The offspring regained the weight during the maturation phase. It is not known whether tolterodine is excreted in human milk; therefore, tolterodine should not be administered during nursing. A decision should be made whether to discontinue nursing or to discontinue tolterodine in nursing mothers.
Pediatric Use
Efficacy in the pediatric population has not been demonstrated.
Two pediatric phase 3 randomized, placebo-controlled, double-blind, 12 week studies were conducted using tolterodine extended-release capsules. A total of 710 pediatric patients (486 on tolterodine extended-release capsules and 224 on placebo) aged 5 to 10 years with urinary frequency and urge urinary incontinence were studied. The percentage of patients with urinary tract infections was higher in patients treated with tolterodine extended-release capsules (6.6%) compared to patients who received placebo (4.5%). Aggressive, abnormal, and hyperactive behavior and attention disorders occurred in 2.9% of children treated with tolterodine extended-release capsules compared to 0.9% of children treated with placebo.
Geriatric Use
Of the 1120 patients who were treated in the four Phase 3, 12 week clinical studies of tolterodine, 474 (42%) were 65 to 91 years of age. No overall differences in safety were observed between the older and younger patients (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations).
ADVERSE REACTIONS
The Phase 2 and 3 clinical trial program for tolterodine tablets included 3071 patients who were treated with tolterodine (N = 2133) or placebo (N = 938). The patients were treated with 1, 2, 4, or 8 mg/day for up to 12 months. No differences in the safety profile of tolterodine were identified based on age, gender, race, or metabolism.
The data described below reflect exposure to tolterodine 2 mg BID in 986 patients and to placebo in 683 patients exposed for 12 weeks in five Phase 3, controlled clinical studies. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and approximating rates.
Sixty-six percent of patients receiving tolterodine 2 mg BID reported adverse events versus 56% of placebo patients. The most common adverse events reported by patients receiving tolterodine were dry mouth, headache, constipation, vertigo/dizziness, and abdominal pain. Dry mouth, constipation, abnormal vision (accommodation abnormalities), urinary retention, and xerophthalmia are expected side effects of antimuscarinic agents.
Dry mouth was the most frequently reported adverse event for patients treated with tolterodine 2 mg BID in the Phase 3 clinical studies, occurring in 34.8% of patients treated with tolterodine and 9.8% of placebo-treated patients. One percent of patients treated with tolterodine discontinued treatment due to dry mouth.
The frequency of discontinuation due to adverse events was highest during the first 4 weeks of treatment. Seven percent of patients treated with tolterodine 2 mg BID discontinued treatment due to adverse events versus 6% of placebo patients. The most common adverse events leading to discontinuation of tolterodine were dizziness and headache.
Three percent of patients treated with tolterodine 2 mg BID reported a serious adverse event versus 4% of placebo patients. Significant ECG changes in QT and QTc have not been demonstrated in clinical-study patients treated with tolterodine 2 mg BID. Table 5 lists the adverse events reported in 1% or more of the patients treated with tolterodine 2 mg BID in the 12 week studies. The adverse events are reported regardless of causality.
|
|||
|
Body System |
Adverse Event |
% TolterodineN = 986 |
% PlaceboN = 683 |
|
Autonomic Nervous |
Accommodation abnormal |
2 |
1 |
|
Dry mouth |
35 |
10 |
|
|
General |
Chest pain |
2 |
1 |
|
Fatigue |
4 |
3 |
|
|
Headache |
7 |
5 |
|
|
Influenza-like symptoms |
3 |
2 |
|
|
Central/Peripheral Nervous |
Vertigo/dizziness |
5 |
3 |
|
Gastrointestinal |
Abdominal pain |
5 |
3 |
|
Constipation |
7 |
4 |
|
|
Diarrhea |
4 |
3 |
|
|
Dyspepsia |
4 |
1 |
|
|
Urinary |
Dysuria |
2 |
1 |
|
Skin/Appendages |
Dry skin |
1 |
0 |
|
Musculoskeletal |
Arthralgia |
2 |
1 |
|
Vision |
Xerophthalmia |
3 |
2 |
|
Psychiatric |
Somnolence |
3 |
2 |
|
Metabolic/Nutritional |
Weight gain |
1 |
0 |
|
Resistance Mechanism |
Infection |
1 |
0 |
Postmarketing Surveillance
The following events have been reported in association with tolterodine use in worldwide postmarketing experience: General: anaphylaxis and angioedema; Cardiovascular: tachycardia, palpitations, peripheral edema; Central/Peripheral Nervous: confusion, disorientation, memory impairment, hallucinations.
Reports of aggravation of symptoms of dementia (e.g., confusion, disorientation, delusion) have been reported after tolterodine therapy was initiated in patients taking cholinesterase inhibitors for the treatment of dementia.
Because these spontaneously reported events are from the worldwide postmarketing experience, the frequency of events and the role of tolterodine in their causation cannot be reliably determined.
OVERDOSAGE
A 27 month-old child who ingested 5 to 7 tolterodine tablets 2 mg was treated with a suspension of activated charcoal and was hospitalized overnight with symptoms of dry mouth. The child fully recovered.
Management of Overdosage
Overdosage with tolterodine can potentially result in severe central anticholinergic effects and should be treated accordingly.
ECG monitoring is recommended in the event of overdosage. In dogs, changes in the QT interval (slight prolongation of 10% to 20%) were observed at a suprapharmacologic dose of 4.5 mg/kg, which is about 68 times higher than the recommended human dose. In clinical trials of normal volunteers and patients, QT interval prolongation was observed with tolterodine immediate-release at doses up to 8 mg (4 mg BID) and higher doses were not evaluated (see PRECAUTIONS, Patients With Congenital or Acquired QT Prolongation).
DOSAGE AND ADMINISTRATION
The initial recommended dose of tolterodine tartrate tablets is 2 mg twice daily. The dose may be lowered to 1 mg twice daily based on individual response and tolerability. For patients with significantly reduced hepatic or renal function or who are currently taking drugs that are potent inhibitors of CYP3A4, the recommended dose of tolterodine tartrate is 1 mg twice daily (see PRECAUTIONS, General; PRECAUTIONS, Reduced Hepatic and Renal Function, and PRECAUTIONS, Drug Interactions).
HOW SUPPLIED
Tolterodine Tartrate Tablets, 1 mg, are available as white, round, unscored, biconvex, film-coated tablets, debossed with “93” on one side and “0010” on the other side containing 1 mg tolterodine tartrate, packaged in bottles of 30 (NDC 71205-319-30), 60 (NDC 71205-319-60) and 90 (NDC 71205-319-90) tablets.
PHARMACIST: Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature].
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured In India By:
Cipla Ltd.
Goa, India
Manufactured For:
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Rev. D 4/2015
TOLTERODINE TARTRATE TABLETS
PATIENT INFORMATION
TOLTERODINE (Tol-TER-oh-deen) TARTRATE (TAHR-trat) TABLETS
Read the Patient Information that comes with tolterodine tartrate tablets before you start using them and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or your treatment. Only your doctor can determine if treatment with tolterodine tartrate tablets is right for you.
What are tolterodine tartrate tablets?
Tolterodine tartrate tablets are a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
- •
- Urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- •
- Urgency: a strong need to urinate right away
- •
- Frequency: urinating often
Tolterodine tartrate extended-release capsules did not help the symptoms of overactive bladder when studied in children.
What is overactive bladder?
Overactive bladder happens when you cannot control your bladder muscle. When the muscle contracts too often or cannot be controlled, you get symptoms of overactive bladder, which are leakage of urine (urge urinary incontinence), needing to urinate right away (urgency), and needing to urinate often (frequency).
Who should not take tolterodine tartrate tablets?
Do not take tolterodine tartrate tablets if you:
- •
- Are not able to empty your bladder (urinary retention)
- •
- Have delayed or slow emptying of your stomach (gastric retention)
- •
- Have an eye problem called "uncontrolled narrow-angle glaucoma"
- •
- Are allergic to tolterodine tartrate tablets or to any of its ingredients. See the end of this leaflet for a complete list of ingredients
- •
- Are allergic to Toviaz® (fesoterodine fumarate) which contains fesoterodine
What should I tell my doctor before starting tolterodine tartrate tablets?
Before starting tolterodine tartrate tablets, tell your doctor about all of your medical and other conditions that may affect the use of tolterodine tartrate tablets, including:
- •
- Stomach or intestinal problems or problems with constipation
- •
- Problems emptying your bladder or if you have a weak urine stream
- •
- Treatment for an eye problem called narrow-angle glaucoma
- •
- Liver problems
- •
- Kidney problems
- •
- A condition called myasthenia gravis
- •
- If you or any family members have a rare heart condition called QT prolongation (long QT syndrome)
- •
- If you are pregnant or trying to become pregnant. It is not known if tolterodine tartrate tablets could harm your unborn baby.
- •
- If you are breastfeeding. It is not known if tolterodine tartrate tablets pass into your breast milk or if they can harm your baby. Talk to your doctor about the best way to feed your baby if you take tolterodine tartrate tablets.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Other medicines can affect how your body handles tolterodine tartrate tablets. Your doctor may use a lower dose of tolterodine tartrate tablets if you are taking:
- •
- Certain medicines for fungus or yeast infections
- •
- Certain medicines for bacterial infections
- •
- Sandimmune® (cyclosporine) or Velban® (vinblastine)
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them with you to show your doctor or pharmacist each time you get a new medicine.
How should I take tolterodine tartrate tablets?
- •
- Take tolterodine tartrate tablets exactly as your doctor tells you to take them.
- •
- Your doctor will tell you how many tolterodine tartrate tablets to take and when to take them.
- •
- Do not change your dose unless told to do so by your doctor.
- •
- You can take tolterodine tartrate tablets with or without food.
- •
- Take tolterodine tartrate tablets at the same times each day.
- •
- If you miss a dose of tolterodine tartrate tablets, just take your next regular dose at your next regular time. Do not try to make up for your missed dose.
- •
- If you take too many tolterodine tartrate tablets, call your doctor, or go to the hospital emergency room right away.
What should I avoid while taking tolterodine tartrate tablets?
Medicines like tolterodine tartrate tablets can cause blurred vision, dizziness, and drowsiness. Do not drive, operate machinery, or do other dangerous activities until you know how tolterodine tartrate tablets affect you.
What are possible side effects of tolterodine tartrate tablets?
Tolterodine tartrate tablets may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking tolterodine tartrate tablets and get emergency medical help right away.
The most common side effects with tolterodine tartrate tablets are:
- •
- Dry mouth
- •
- Dizziness
- •
- Headache
- •
- Stomach pain
- •
- Constipation
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all the side effects with tolterodine tartrate tablets. For a complete list, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How do I store tolterodine tartrate tablets?
- •
- Store tolterodine tartrate tablets at 20º to 25ºC (68º to 77ºF).
- •
- Keep them in a dry place.
Keep tolterodine tartrate tablets and all medicines out of the reach of children.
General information about tolterodine tartrate tablets
Medicines are sometimes prescribed for conditions that are not mentioned in the Patient Information leaflet. Only use tolterodine tartrate tablets the way your doctor tells you. Do not give tolterodine tartrate tablets to other people even if they have the same symptoms you have. They may harm them.
This leaflet summarizes the most important information about tolterodine tartrate tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about tolterodine tartrate tablets that is written for health professionals. For more information about tolterodine tartrate tablets, call Teva Pharmaceuticals Medical Affairs at 1-888-838-2872.
What are the ingredients in tolterodine tartrate tablets?
Active ingredients: tolterodine tartrate
Inactive ingredients: corn starch, croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, sodium stearyl fumarate, and titanium dioxide.
Manufactured In India By:
Cipla Ltd.
Goa, India
Manufactured For:
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Repackaged by:
Proficient Rx LP
Thousand Oaks, CA 91320
Rev. B 4/2015
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.