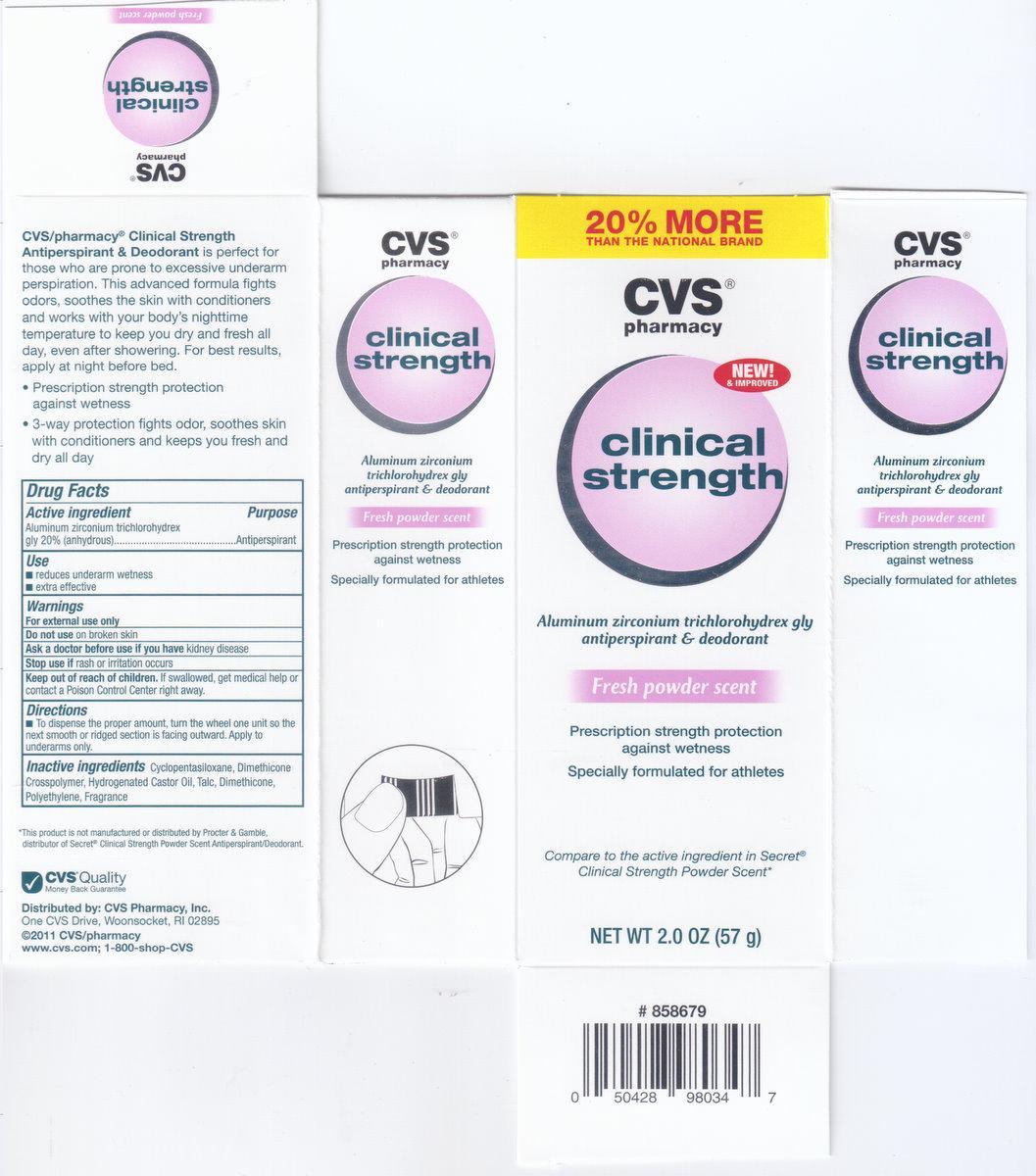
CVS PHARMACY CLINICAL STRENGTH- aluminum zirconium trichlorohydrex gly stick
CVS pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients: Aluminum Zirconium Trichlorohydrex Gly 20% (anhydrous)
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
Use
- Reduces underarm wetness
- Extra effective
Warning:
For external use only.
Do not use on broken skin.
Ask doctor before you use if you have kidney disease.
Stop use if rash or irritation occurs.
Directions:
To dispense the proper dosage, turn the wheel one unit so the smooth or ribbed section is facing to the outside.Apply to underarms only.
Ingredients:Cyclopentasiloxane, Dimethicone crosspolymer, Hydrogenated Castor Oil, Talc, Dimethicone, Polyethylene, Fragrance