FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Hyperlipidemia and Mixed Dyslipidemia
Rosuvastatin tablets are indicated as adjunctive therapy to diet to reduce elevated Total-C, LDL-C, ApoB, nonHDL-C, and triglycerides and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol when response to diet and nonpharmacological interventions alone has been inadequate.
1.2 Pediatric Patients with Familial Hypercholesterolemia
Rosuvastatin tablets are indicated as an adjunct to diet to:
- reduce Total-C, LDL-C and ApoB levels in children and adolescents 8 to 17 years of age with heterozygous familial hypercholesterolemia if after an adequate trial of diet therapy the following findings are present: LDL-C >190 mg/dL, or >160 mg/dL along with a positive family history of premature cardiovascular disease (CVD) or two or more other CVD risk factors.
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
1.3 Hypertriglyceridemia
Rosuvastatin tablets are indicated as adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia.
1.4 Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia)
Rosuvastatin tablets are indicated as an adjunct to diet for the treatment of adult patients with primary dysbetalipoproteinemia (Type III Hyperlipoproteinemia).
1.5 Adult Patients with Homozygous Familial Hypercholesterolemia
Rosuvastatin tablets are indicated as adjunctive therapy to other lipid-lowering treatments (e.g., LDL apheresis) or alone if such treatments are unavailable to reduce LDL-C, Total-C, and ApoB in adult patients with homozygous familial hypercholesterolemia.
1.6 Slowing of the Progression of Atherosclerosis
Rosuvastatin tablets are indicated as adjunctive therapy to diet to slow the progression of atherosclerosis in adult patients as part of a treatment strategy to lower Total-C and LDL-C to target levels.
1.7 Primary Prevention of Cardiovascular Disease
In individuals without clinically evident coronary heart disease but with an increased risk of cardiovascular disease based on age ≥50 years old in men and ≥60 years old in women, hsCRP ≥2 mg/L, and the presence of at least one additional cardiovascular disease risk factor such as hypertension, low HDL-C, smoking, or a family history of premature coronary heart disease, rosuvastatin tablets are indicated to:
- reduce the risk of stroke
- reduce the risk of myocardial infarction
- reduce the risk of arterial revascularization procedures
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
The dose range for rosuvastatin tablets in adults is 5 to 40 mg orally once daily. The usual starting dose is 10 to 20 mg once daily. The usual starting dose in adult patients with homozygous familial hypercholesterolemia is 20 mg once daily.
The maximum rosuvastatin tablets dose of 40 mg should be used only for those patients who have not achieved their LDL-C goal utilizing the 20 mg dose [see Warnings and Precautions (5.1)].
Rosuvastatin tablets can be administered as a single dose at any time of day, with or without food. The tablet should be swallowed whole.
When initiating rosuvastatin tablets therapy or switching from another HMG-CoA reductase inhibitor therapy, the appropriate rosuvastatin tablets starting dose should first be utilized, and only then titrated according to the patient’s response and individualized goal of therapy.
After initiation or upon titration of rosuvastatin tablets, lipid levels should be analyzed within 2 to 4 weeks and the dosage adjusted accordingly.
2.2 Pediatric Dosing
In heterozygous familial hypercholesterolemia, the recommended dose range is 5 to 10 mg orally once daily in patients 8 to less than 10 years of age, and 5 to 20 mg orally once daily in patients 10 to 17 years of age.
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.3 Dosing in Asian Patients
In Asian patients, consider initiation of rosuvastatin tablets therapy with 5 mg once daily due to increased rosuvastatin plasma concentrations. The increased systemic exposure should be taken into consideration when treating Asian patients not adequately controlled at doses up to 20 mg/day [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
2.4 Use with Concomitant Therapy
Patients taking cyclosporine and darolutamide
The dose of rosuvastatin tablets should not exceed 5 mg once daily
[see
Warnings and Precautions (5.1),
Drug Interactions (7.1),
Drug Interactions (7.4) and
Clinical Pharmacology (12.3)].
Patients taking gemfibrozil
Avoid concomitant use of rosuvastatin tablets with gemfibrozil. If concomitant use cannot be avoided, initiate rosuvastatin tablets at 5 mg once daily. The dose of rosuvastatin tablets should not exceed 10 mg once daily
[see
Warnings and Precautions (5.1),
Drug Interactions (7.2) and
Clinical Pharmacology (12.3)].
Patients taking regorafenib
Concomitant use of rosuvastatin tablets and regorafenib, the dose of rosuvastatin tablets should not exceed 10 mg once daily
[see
Warnings and Precautions (5.1),
Drug Interactions (7.5) and
Clinical Pharmacology (12.3)].
Patients taking atazanavir and ritonavir, lopinavir and ritonavir, simeprevir or combination of dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, sofosbuvir/velpatasvir and glecaprevir/pibrentasvir
Initiate rosuvastatin tablets therapy with 5 mg once daily. The dose of rosuvastatin tablets should not exceed 10 mg once daily
[see
Warnings and Precautions (5.1),
Drug Interactions (7.3) and
Clinical Pharmacology (12.3)].
2.5 Dosing in Patients with Severe Renal Impairment
For patients with severe renal impairment (CL cr <30 mL/min/1.73 m 2) not on hemodialysis, dosing of rosuvastatin tablets should be started at 5 mg once daily and not exceed 10 mg once daily [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
Rosuvastatin Tablets USP are available containing 5 mg, 10 mg, 20 mg or 40 mg of rosuvastatin.
5 mg: Pink, oval shaped, biconvex film-coated tablets debossed with ‘I’ on one side and ‘29’ on the other side.
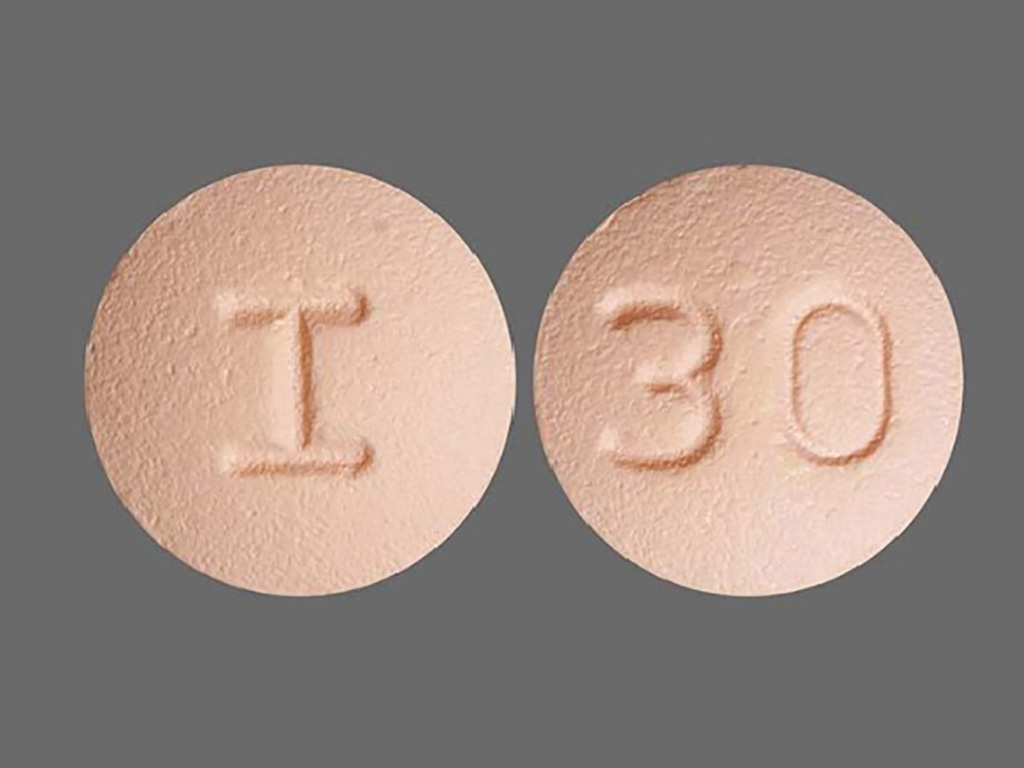
10 mg: Pink, round, biconvex film-coated tablets debossed with ‘I’ on one side and ‘30’ on the other side.
20 mg: Pink, round, biconvex film-coated tablets debossed with ‘I’ on one side and ‘31’ on the other side.
40 mg: Pink, oval shaped, biconvex film-coated tablets debossed with ‘I’ on one side and ‘32’ on the other side.
4 CONTRAINDICATIONS
Rosuvastatin tablets are contraindicated in the following conditions:
- Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin [see Adverse Reactions (6.1)].
- Patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels [see Warnings and Precautions (5.3)].
- Pregnancy [see Use in Specific Populations (8.1, 8.3)].
- Lactation. Limited data indicate that rosuvastatin is present in human milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require rosuvastatin treatment should not breastfeed their infants [see Use in Specific Populations (8.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg).
Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age ≥ 65 years, inadequately treated hypothyroidism, renal impairment).
The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of gemfibrozil, some other lipid-lowering therapies (other fibrates or niacin), cyclosporine, darolutamide, regorafenib, atazanavir/ritonavir, lopinavir/ritonavir, simeprevir or combination of sofosbuvir/velpatasvir/voxilaprevir, dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, glecaprevir/pibrentasvir, all combinations with ledipasvir (including ledipasvir/sofosbuvir) [see Dosage and Administration (2) and Drug Interactions (7)]. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine [see Drug Interactions (7.9)].
Rosuvastatin therapy should be discontinued if markedly elevated creatine kinase levels occur or myopathy is diagnosed or suspected. Rosuvastatin therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures).
All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing rosuvastatin.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Consider risk of IMNM carefully prior to initiation of a different statin. If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM.
5.3 Liver Enzyme Abnormalities
It is recommended that liver enzyme tests be performed before the initiation of rosuvastatin, and if signs or symptoms of liver injury occur.
Increases in serum transaminases [AST (SGOT) or ALT (SGPT)] have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy. There were two cases of jaundice, for which a relationship to rosuvastatin therapy could not be determined, which resolved after discontinuation of therapy. There were no cases of liver failure or irreversible liver disease in these trials.
In a pooled analysis of placebo-controlled trials, increases in serum transaminases to >3 times the upper limit of normal occurred in 1.1% of patients taking rosuvastatin versus 0.5% of patients treated with placebo.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with rosuvastatin, promptly interrupt therapy. If an alternate etiology is not found, do not restart rosuvastatin.
Rosuvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease [see Clinical Pharmacology (12.3)]. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of rosuvastatin [see Contraindications (4)].
5.4 Concomitant Coumarin Anticoagulants
Caution should be exercised when anticoagulants are given in conjunction with rosuvastatin because of its potentiation of the effect of coumarin-type anticoagulants in prolonging the prothrombin time/INR. In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs [see Drug Interactions (7.6)].
5.5 Proteinuria and Hematuria
In the rosuvastatin clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin treated patients. These findings were more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator HMG-CoA reductase inhibitors, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, a dose reduction should be considered for patients on rosuvastatin therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
5.6 Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus [see Adverse Reactions (6.1)].
Although clinical studies have shown that rosuvastatin alone does not reduce basal plasma cortisol concentration or impair adrenal reserve, caution should be exercised if rosuvastatin is administered concomitantly with drugs that may decrease the levels or activity of endogenous steroid hormones such as ketoconazole, spironolactone, and cimetidine.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)]
- Liver enzyme abnormalities [see Warnings and Precautions (5.2)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
In the rosuvastatin controlled clinical trials database (placebo or active-controlled) of 5394 patients with a mean treatment duration of 15 weeks, 1.4% of patients discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were:
- myalgia
- abdominal pain
- nausea
The most commonly reported adverse reactions (incidence ≥ 2%) in the rosuvastatin controlled clinical trial database of 5394 patients were:
- headache
- myalgia
- abdominal pain
- asthenia
- nausea
Adverse reactions reported in ≥ 2% of patients in placebo-controlled clinical studies and at a rate greater than placebo are shown in Table 1. These studies had a treatment duration of up to 12 weeks.
|
||||||
|
Adverse Reactions |
Rosuvastatin 5 mg N=291 |
Rosuvastatin 10 mg N=283 |
Rosuvastatin 20 mg N=64 |
Rosuvastatin 40 mg N=106 |
Total Rosuvastatin 5 mg to 40 mg N=744 |
Placebo N=382 |
|
Headache |
5.5 |
4.9 |
3.1 |
8.5 |
5.5 |
5.0 |
|
Nausea |
3.8 |
3.5 |
6.3 |
0 |
3.4 |
3.1 |
|
Myalgia |
3.1 |
2.1 |
6.3 |
1.9 |
2.8 |
1.3 |
|
Asthenia |
2.4 |
3.2 |
4.7 |
0.9 |
2.7 |
2.6 |
|
Constipation |
2.1 |
2.1 |
4.7 |
2.8 |
2.4 |
2.4 |
Other adverse reactions reported in clinical studies were abdominal pain, dizziness, hypersensitivity (including rash, pruritus, urticaria, and angioedema) and pancreatitis. The following laboratory abnormalities have also been reported: dipstick-positive proteinuria and microscopic hematuria [see Warnings and Precautions (5.5)]; elevated creatine phosphokinase, transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and bilirubin; and thyroid function abnormalities.
In a clinical trial, involving 981 participants treated with rosuvastatin 40 mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years, 5.6% of subjects treated with rosuvastatin versus 2.8% of placebo-treated subjects discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: myalgia, hepatic enzyme increased, headache, and nausea [see Clinical Studies (14.8)].
Adverse reactions reported in ≥ 2% of patients and at a rate greater than placebo are shown in Table 2.
|
Adverse Reactions |
Rosuvastatin 40 mg N=700 |
Placebo N=281 |
|
Myalgia |
12.7 |
12.1 |
|
Arthralgia |
10.1 |
7.1 |
|
Headache |
6.4 |
5.3 |
|
Dizziness |
4.0 |
2.8 |
|
Increased CPK |
2.6 |
0.7 |
|
Abdominal pain |
2.4 |
1.8 |
|
ALT >3x ULN † |
2.2 |
0.7 |
In a clinical trial, 17,802 participants were treated with rosuvastatin 20 mg (n=8901) or placebo (n=8901) for a mean duration of 2 years. A higher percentage of rosuvastatin-treated patients versus placebo-treated patients, 6.6% and 6.2%, respectively, discontinued study medication due to an adverse event, irrespective of treatment causality. Myalgia was the most common adverse reaction that led to treatment discontinuation.
There was a significantly higher frequency of diabetes mellitus reported in patients taking rosuvastatin (2.8%) versus patients taking placebo (2.3%). Mean HbA1c was significantly increased by 0.1% in rosuvastatin-treated patients compared to placebo-treated patients. The number of patients with a HbA1c > 6.5% at the end of the trial was significantly higher in rosuvastatin-treated versus placebo-treated patients [see Warnings and Precautions (5.6) and Clinical Studies (14.9)].
Adverse reactions reported in ≥ 2% of patients and at a rate greater than placebo are shown in Table 3.
|
||
|
Adverse Reactions |
Rosuvastatin 20 mg N=8901 |
Placebo N=8901 |
|
Myalgia |
7.6 |
6.6 |
|
Arthralgia |
3.8 |
3.2 |
|
Constipation |
3.3 |
3.0 |
|
Diabetes mellitus |
2.8 |
2.3 |
|
Nausea |
2.4 |
2.3 |
Pediatric Patients with Heterozygous Familial Hypercholesterolemia
In a 12-week controlled study in boys and postmenarcheal girls 10 to 17 years of age with heterozygous familial hypercholesterolemia with rosuvastatin 5 to 20 mg daily
[see
Use in Specific Populations (8.4) and
Clinical Studies (14.7)],
elevations in serum creatine phosphokinase (CK) >10 x ULN were observed more frequently in rosuvastatin compared with placebo-treated children. Four of 130 (3%) children treated with rosuvastatin (2 treated with 10 mg and 2 treated with 20 mg) had increased CK >10 x ULN, compared to 0 of 46 children on placebo.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of rosuvastatin: arthralgia, fatal and non-fatal hepatic failure, hepatitis, jaundice, thrombocytopenia, depression, sleep disorders (including insomnia and nightmares), peripheral neuropathy, interstitial lung disease and gynecomastia. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.2)].
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, and confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
7 DRUG INTERACTIONS
7.1 Cyclosporine
Cyclosporine increased rosuvastatin exposure and may result in increased risk of myopathy. Therefore, in patients taking cyclosporine, the dose of rosuvastatin should not exceed 5 mg once daily [see Dosage and Administration (2.4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Gemfibrozil
Gemfibrozil significantly increased rosuvastatin exposure. Due to an observed increased risk of myopathy/rhabdomyolysis, combination therapy with rosuvastatin and gemfibrozil should be avoided. If used together, the dose of rosuvastatin should not exceed 10 mg once daily [see Clinical Pharmacology (12.3)].
7.3 Anti-viral Medications
Coadministration of rosuvastatin with certain anti-viral drugs has differing effects on rosuvastatin exposure and may increase risk of myopathy.
The combination of sofosbuvir/velpatasvir/voxilaprevir which are anti-Hepatitis C virus (anti-HCV) drugs, increases rosuvastatin exposure. Similarly, the combination of ledipasvir/sofosbuvir may significantly increase rosuvastatin exposure. For these combinations of anti-HCV drugs, concomitant use with rosuvastatin is not recommended.
Simeprevir and combinations of dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, sofosbuvir/velpatasvir and glecaprevir/pibrentasvir which are anti-HCV drugs, increase rosuvastatin exposure. Combinations of atazanavir/ritonavir and lopinavir/ritonavir, which are anti-HIV-1 drugs, increase rosuvastatin exposure [see Table 4 – Clinical Pharmacology (12.3)]. For these anti-viral drugs, the dose of rosuvastatin should not exceed 10 mg once daily.
The combinations of fosamprenavir/ritonavir or tipranavir/ritonavir, which are anti-HIV-1 drugs, produce little or no change in rosuvastatin exposure. No dose adjustment is needed for concomitant use with these combinations [see Dosage and Administration (2.4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.4 Darolutamide
Darolutamide increased rosuvastatin exposure more than 5 fold. Therefore, in patients taking darolutamide, the dose of rosuvastatin should not exceed 5 mg once daily [see Dosage and Administration (2.4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.5 Regorafenib
Regorafenib increased rosuvastatin exposure and may increase the risk of myopathy. If used together, the dose of rosuvastatin should not exceed 10 mg once daily [see Dosage and Administration (2.4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.6 Coumarin Anticoagulants
Rosuvastatin significantly increased INR in patients receiving coumarin anticoagulants. Therefore, caution should be exercised when coumarin anticoagulants are given in conjunction with rosuvastatin. In patients taking coumarin anticoagulants and rosuvastatin concomitantly, INR should be determined before starting rosuvastatin and frequently enough during early therapy to ensure that no significant alteration of INR occurs [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.7 Niacin
The risk of skeletal muscle effects may be enhanced when rosuvastatin is used in combination with lipid-modifying doses (≥1 g/day) of niacin; caution should be used when prescribing with rosuvastatin [see Warnings and Precautions (5.1)].
7.8 Fenofibrate
When rosuvastatin was coadministered with fenofibrate, no clinically significant increase in the AUC of rosuvastatin or fenofibrate was observed. Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concomitant use of fenofibrates, caution should be used when prescribing fenofibrates with rosuvastatin [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.9 Colchicine
Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Rosuvastatin is contraindicated for use in pregnant women since safety in pregnant women has not been established and there is no apparent benefit to therapy with rosuvastatin during pregnancy. Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, rosuvastatin may cause fetal harm when administered to pregnant women. Rosuvastatin should be discontinued as soon as pregnancy is recognized
[see
Contraindications (4)].
Limited published data on the use of rosuvastatin are insufficient to determine a drug-associated risk of major congenital malformations or miscarriage. In animal reproduction studies, there were no adverse developmental effects with oral administration of rosuvastatin during organogenesis at systemic exposures equivalent to a maximum recommended human dose (MRHD) of 40 mg/day in rats or rabbits (based on AUC and body surface area, respectively). In rats and rabbits, decreased pup/fetal survival occurred at 12 times and equivalent, respectively, to the MRHD of 40 mg/day
[see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Limited published data on rosuvastatin have not shown an increased risk of major congenital malformations or miscarriage. Rare reports of congenital anomalies have been received following intrauterine exposure to other statins. In a review of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or lovastatin, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate to exclude a ≥3 to 4-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Animal Data
Rosuvastatin crosses the placenta in rats and rabbits and is found in fetal tissue and amniotic fluid at 3% and 20%, respectively, of the maternal plasma concentration following a single 25 mg/kg oral gavage dose on gestation day 16 in rats. A higher fetal tissue distribution (25% maternal plasma concentration) was observed in rabbits after a single oral gavage dose of 1 mg/kg on gestation day 18.
Rosuvastatin administration did not indicate a teratogenic effect in rats at ≤25 mg/kg/day or in rabbits ≤3 mg/kg/day (doses equivalent to the MRHD of 40 mg/day based on AUC and body surface area, respectively).
In female rats given 5, 15 and 50 mg/kg/day before mating and continuing through to gestation day 7 resulted in decreased fetal body weight (female pups) and delayed ossification at 50 mg/kg/day (10 times the human exposure at the MRHD dose of 40 mg/day based on AUC).
In pregnant rats given 2, 10 and 50 mg/kg/day of rosuvastatin from gestation day 7 through lactation day 21 (weaning), decreased pup survival occurred at 50 mg/kg/day (dose equivalent to 12 times the MRHD of 40 mg/day based body surface area).
In pregnant rabbits given 0.3, 1, and 3 mg/kg/day of rosuvastatin from gestation day 6 to day 18, decreased fetal viability and maternal mortality was observed at 3 mg/kg/day (dose equivalent to the MRHD of 40 mg/day based on body surface area).
8.2 Lactation
Risk Summary
Rosuvastatin use is contraindicated during breastfeeding
[see
Contraindications (4)].
Limited data indicate that rosuvastatin is present in human milk. There is no available information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. Because of the potential for serious adverse reactions in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with rosuvastatin.
8.3 Females and Males of Reproductive Potential
Contraception
Rosuvastatin may cause fetal harm when administered to a pregnant woman
[see
Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with rosuvastatin.
8.4 Pediatric Use
In children and adolescents 8 to 17 years of age with heterozygous familial hypercholesterolemia, the safety and effectiveness of rosuvastatin as an adjunct to diet to reduce total cholesterol, LDL-C, and ApoB levels when, after an adequate trial of diet therapy, LDL-C exceeds 190 mg/dL or when LDL-C exceeds 160 mg/dL and there is a positive family history of premature CVD or two or more other CVD risk factors, were established in one controlled trial and in one open-label, uncontrolled trial [see Clinical Studies (14.7)]. The long-term efficacy of rosuvastatin therapy initiated in childhood to reduce morbidity and mortality in adulthood has not been established.
The safety and effectiveness of rosuvastatin in children and adolescents 10 to 17 years of age with heterozygous familial hypercholesterolemia were evaluated in a controlled clinical trial of 12 weeks duration followed by 40 weeks of open-label exposure. Patients treated with 5 mg, 10 mg, and 20 mg daily rosuvastatin had an adverse experience profile generally similar to that of patients treated with placebo. There was no detectable effect of rosuvastatin on growth, weight, BMI (body mass index), or sexual maturation [see Clinical Studies (14.7)] in children and adolescents (10 to 17 years of age).
Rosuvastatin has not been studied in controlled clinical trials involving prepubertal patients or patients younger than 10 years of age with heterozygous familial hypercholesterolemia. However, the safety and effectiveness of rosuvastatin were evaluated in a two year open-label uncontrolled trial that included children and adolescents 8 to 17 years of age with heterozygous familial hypercholesterolemia [see Clinical Studies (14.7)]. The safety and efficacy of rosuvastatin in lowering LDL-C appeared generally consistent with that observed for adult patients, despite limitations of the uncontrolled study design.
Although not all adverse reactions identified in the adult population have been observed in clinical trials of children and adolescent patients, the same warnings and precautions for adults should be considered for children and adolescents. Adolescent females should be counseled on appropriate contraceptive methods while on rosuvastatin therapy [see Use in Specific Populations (8.1)].
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Of the 10,275 patients in clinical studies with rosuvastatin, 3159 (31%) were 65 years and older, and 698 (6.8%) were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients are at higher risk of myopathy and rosuvastatin should be prescribed with caution in the elderly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Rosuvastatin exposure is not influenced by mild to moderate renal impairment (CL cr ≥ 30 mL/min/1.73 m 2). Exposure to rosuvastatin is increased to a clinically significant extent in patients with severe renal impairment (CL cr <30 mL/min/1.73 m 2) who are not receiving hemodialysis and dose adjustment is required [see Dosage and Administration (2.5), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Rosuvastatin is contraindicated in patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels. Chronic alcohol liver disease is known to increase rosuvastatin exposure; rosuvastatin should be used with caution in these patients [see Contraindications (4), Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
8.8 Asian Patients
Pharmacokinetic studies have demonstrated an approximate 2-fold increase in median exposure to rosuvastatin in Asian subjects when compared with Caucasian controls. Rosuvastatin dosage should be adjusted in Asian patients [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no specific treatment in the event of overdose. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Hemodialysis does not significantly enhance clearance of rosuvastatin.
11 DESCRIPTION
Rosuvastatin calcium is a synthetic lipid-lowering agent for oral administration.
The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt with the following structural formula:
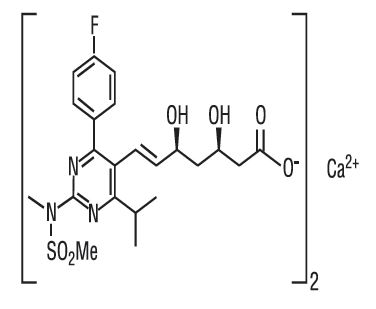
The empirical formula for rosuvastatin calcium is (C 22H 27FN 3O 6S) 2Ca and the molecular weight is 1001.14. Rosuvastatin calcium USP is a white to off-white powder that is sparingly soluble in water and methanol, and slightly soluble in ethanol. Rosuvastatin calcium is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.0.
Rosuvastatin tablets USP for oral administration contain 5 mg, 10 mg, 20 mg or 40 mg of rosuvastatin and the following inactive ingredients: crospovidone, dibasic calcium phosphate anhydrous, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
Meets USP Dissolution Test-2.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rosuvastatin is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol. In vivo studies in animals, and in vitro studies in cultured animal and human cells have shown rosuvastatin to have a high uptake into, and selectivity for, action in the liver, the target organ for cholesterol lowering. In in vivo and in vitro studies, rosuvastatin produces its lipid-modifying effects in two ways. First, it increases the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL. Second, rosuvastatin inhibits hepatic synthesis of VLDL, which reduces the total number of VLDL and LDL particles.
12.2 Pharmacodynamics
Rosuvastatin dose dependently reduces elevated LDL-cholesterol and reduces total cholesterol and triglycerides and increases HDL-cholesterol [see Clinical Studies (14)]. A therapeutic response to rosuvastatin is evident within 1 week of commencing therapy and 90% of maximum response is usually achieved in 2 weeks. The maximum response is usually achieved by 4 weeks and is maintained after that. Individualization of drug dosage should be based on the therapeutic response [see Dosage and Administration (2)].
12.3 Pharmacokinetics
Absorption
In clinical pharmacology studies in man, peak plasma concentrations of rosuvastatin were reached 3 to 5 hours following oral dosing. Both C
max and AUC increased in approximate proportion to rosuvastatin dose. The absolute bioavailability of rosuvastatin is approximately 20%.
Administration of rosuvastatin with food did not affect the AUC of rosuvastatin.
The AUC of rosuvastatin does not differ following evening or morning drug administration.
Distribution
Mean volume of distribution at steady-state of rosuvastatin is approximately 134 liters. Rosuvastatin is 88% bound to plasma proteins, mostly albumin. This binding is reversible and independent of plasma concentrations.
Elimination
Rosuvastatin is primarily eliminated by excretion in the feces. The elimination half-life of rosuvastatin is approximately 19 hours.
Metabolism
Rosuvastatin is not extensively metabolized; approximately 10% of a radiolabeled dose is recovered as metabolite. The major metabolite is N-desmethyl rosuvastatin, which is formed principally by cytochrome P450 \ 2C9, and
in vitro studies have demonstrated that N-desmethyl rosuvastatin has approximately one-sixth to one-half the HMG-CoA reductase inhibitory activity of the parent compound. Overall, greater than 90% of active plasma HMG-CoA reductase inhibitory activity is accounted for by the parent compound.
Excretion
Following oral administration, rosuvastatin and its metabolites are primarily excreted in the feces (90%). After an intravenous dose, approximately 28% of total body clearance was via the renal route, and 72% by the hepatic route.
Specific Populations
Racial or Ethnic Groups
A population pharmacokinetic analysis revealed no clinically relevant differences in pharmacokinetics among Caucasian, Hispanic, and Black or Afro-Caribbean groups. However, pharmacokinetic studies, including one conducted in the U.S., have demonstrated an approximate 2-fold elevation in median exposure (AUC and C
max) in Asian subjects when compared with a Caucasian control group.
Male and Female Patients
There were no differences in plasma concentrations of rosuvastatin between men and women.
Pediatric Patients
In a population pharmacokinetic analysis of two pediatric trials involving patients with heterozygous familial hypercholesterolemia 10 to 17 years of age and 8 to 17 years of age, respectively, rosuvastatin exposure appeared comparable to or lower than rosuvastatin exposure in adult patients.
Geriatric Patients
There were no differences in plasma concentrations of rosuvastatin between the nonelderly and elderly populations (age ≥ 65 years).
Patients with Renal Impairment
Mild to moderate renal impairment (CL
cr ≥ 30 mL/min/1.73 m
2) had no influence on plasma concentrations of rosuvastatin. However, plasma concentrations of rosuvastatin increased to a clinically significant extent (about 3-fold) in patients with severe renal impairment (CL
cr < 30 mL/min/1.73 m
2) not receiving hemodialysis compared with healthy subjects (CL
cr > 80 mL/min/1.73 m
2).
Hemodialysis
Steady-state plasma concentrations of rosuvastatin in patients on chronic hemodialysis were approximately 50% greater compared with healthy volunteer subjects with normal renal function.
Patients with Hepatic Impairment
In patients with chronic alcohol liver disease, plasma concentrations of rosuvastatin were modestly increased.
In patients with Child-Pugh A disease, C max and AUC were increased by 60% and 5%, respectively, as compared with patients with normal liver function. In patients with Child-Pugh B disease, C max and AUC were increased 100% and 21%, respectively, compared with patients with normal liver function.
Drug Interactions Studies
Rosuvastatin clearance is not dependent on metabolism by cytochrome P450 3A4 to a clinically significant extent.
Rosuvastatin is a substrate for certain transporter proteins including the hepatic uptake transporter organic anion-transporting polyprotein 1B1 (OATP1B1) and efflux transporter breast cancer resistance protein (BCRP). Concomitant administration of rosuvastatin with medications that are inhibitors of these transporter proteins (e.g., cyclosporine, certain HIV protease inhibitors) may result in increased rosuvastatin plasma concentrations [see Dosage and Administration (2.4) and Drug Interactions (7.1, 7.3)].
|
Coadministered drug and dosing regimen |
Rosuvastatin |
||
|
Mean Ratio (ratio with/without coadministered drug) No Effect = 1.0 |
|||
|
Dose (mg)* |
Change in AUC |
Change in C max |
|
|
Sofosbuvir/velpatasvir/voxilaprevir (400 mg to 100 mg to 100 mg) + Voxilaprevir (100 mg) once daily for 15 days |
10 mg single dose |
7.39 † (6.68 to 8.18) ‡ |
18.88 † (16.23 to 21.96) ‡ |
|
Cyclosporine – stable dose required (75 mg to 200 mg BID) |
10 mg QD for 10 days |
7.1 † |
11 † |
|
Darolutamide 600 mg BID, 5 days |
5 mg, single dose |
5.2 † |
~5 † |
|
Regorafenib 160 mg OD, 14 days |
5 mg single dose |
3.8 † |
4.6 † |
|
Atazanavir/ritonavir combination
|
10 mg |
3.1 † |
7 † |
|
Simeprevir 150 mg QD, 7 days |
10 mg, single dose |
2.8 † (2.3 to 3.4) ‡ |
3.2 † (2.6 to 3.9) ‡ |
|
Velpatasvir 100 mg once daily |
10 mg single dose |
2.69 † (2.46 to 2.94) ‡ |
2.61 † (2.32 to 2.92) ‡ |
|
Ombitasvir 25 mg/paritaprevir 150 mg/ ritonavir 100 mg + dasabuvir 400 mg BID |
5 mg single dose |
2.59 † (2.09 to 3.21) ‡ |
7.13 † (5.11 to 9.96) ‡ |
|
Elbasvir 50 mg/grazoprevir 200 mg once daily |
10 mg single dose |
2.26 † (1.89 to 2.69) ‡ |
5.49 † (4.29 to 7.04) ‡ |
|
Glecaprevir 400 mg/pibrentasvir 120 mg once daily |
5 mg once daily |
2.15 † (1.88 to 2.46) ‡ |
5.62 † (4.80 to 6.59) ‡ |
|
Lopinavir/ritonavir combination
|
20 mg QD for 7 days |
2.1 † (1.7 to 2.6) ‡ |
5 † (3.4 to 6.4) ‡ |
|
Gemfibrozil 600 mg BID for 7 days |
80 mg |
1.9 † (1.6 to 2.2) ‡ |
2.2 † (1.8 to 2.7) ‡ |
|
Eltrombopag 75 mg QD, 5 days |
10 mg |
1.6 (1.4 to 1.7) ‡ |
2 (1.8 to 2.3) ‡ |
|
Darunavir 600 mg/ritonavir 100 mg BID, 7 days |
10 mg QD for 7 days |
1.5 (1.0 to 2.1) ‡ |
2.4 (1.6 to 3.6) ‡ |
|
Tipranavir/ritonavir combination 500 mg/200 mg BID for 11 days |
10 mg |
1.4 (1.2 to 1.6) ‡ |
2.2 (1.8 to 2.7) ‡ |
|
Dronedarone 400 mg BID |
10 mg |
1.4 | |
|
Itraconazole 200 mg QD, 5 days |
10 mg or 80 mg |
1.4 (1.2 to 1.6) ‡ 1.3 (1.1 to 1.4) ‡ |
1.4 (1.2 to 1.5) ‡ 1.2 (0.9 to 1.4) ‡ |
|
Ezetimibe 10 mg QD, 14 days |
10 mg QD for 14 days |
1.2 (0.9 to 1.6) ‡ |
1.2 (0.8 to 1.6) ‡ |
|
Fosamprenavir/ritonavir 700 mg/100 mg BID for 7 days |
10 mg |
1.1 |
1.5 |
|
Fenofibrate 67 mg TID for 7 days |
10 mg |
↔ |
1.2 (1.1 to 1.3) ‡ |
|
Rifampicin 450 mg QD, 7 days |
20 mg |
↔ | |
|
Aluminum & magnesium hydroxide combination antacid Administered simultaneously Administered 2 hours apart |
40 mg 40 mg |
0.5 † (0.4 to 0.5) ‡ 0.8 (0.7 to 0.9) ‡ |
0.5 † (0.4 to 0.6) ‡ 0.8 (0.7 to 1.0) ‡ |
|
Ketoconazole 200 mg BID for 7 days |
80 mg |
1.0 (0.8 to 1.2) ‡ |
1.0 (0.7 to 1.3) ‡ |
|
Fluconazole 200 mg QD for 11 days |
80 mg |
1.1 (1.0 to 1.3) ‡ |
1.1 (0.9 to 1.4) ‡ |
|
Erythromycin 500 mg QID for 7 days |
80 mg |
0.8 (0.7 to 0.9) ‡ |
0.7 (0.5 to 0.9) ‡ |
QD= Once daily, BID= Twice daily, TID= Three times daily, QID= Four times daily
|
Rosuvastatin Dosage Regimen |
Coadministered Drug |
||
|
Mean Ratio (ratio with/without coadministered drug) No Effect = 1.0 |
|||
|
Name and Dose |
Change in AUC |
Change in C max |
|
|
40 mg QD for 10 days |
Warfarin * 25 mg single dose |
R-Warfarin 1.0 (1.0 to 1.1 † S-Warfarin 1.1 (1.0 to 1.1) † |
R-Warfarin 1.0 (0.9 to 1.0) † S-Warfarin 1.0 (0.9 to 1.1) † |
|
40 mg QD for 12 days |
Digoxin 0.5 mg single dose |
1.0 (0.9 to 1.2) † |
1.0 (0.9 to 1.2) † |
|
40 mg QD for 28 days |
Oral Contraceptive (ethinyl estradiol 0.035 mg & norgestrel 0.180, 0.215 and 0.250 mg) QD for 21 Days |
EE 1.3 (1.2 to 1.3) † NG 1.3 (1.3 to 1.4) † |
EE 1.3 (1.2 to 1.3) † NG 1.2 (1.1 to 1.3) † |
EE = ethinyl estradiol, NG = norgestrel, QD= Once daily
12.5 Pharmacogenomics
Disposition of HMG-CoA reductase inhibitors, including rosuvastatin, involves OATP1B1 and other transporter proteins. Higher plasma concentrations of rosuvastatin have been reported in very small groups of patients (n=3 to 5) who have two reduced function alleles of the gene that encodes OATP1B1 (SLCO1B1 521T > C). The frequency of this genotype (i.e., SLCO1B1 521 C/C) is generally lower than 5% in most racial/ethnic groups. The impact of this polymorphism on efficacy and/or safety of rosuvastatin has not been clearly established.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week carcinogenicity study in rats at dose levels of 2, 20, 60, or 80 mg/kg/day by oral gavage, the incidence of uterine stromal polyps was significantly increased in females at 80 mg/kg/day at systemic exposure 20 times the human exposure at 40 mg/day based on AUC. Increased incidence of polyps was not seen at lower doses.
In a 107-week carcinogenicity study in mice given 10, 60, or 200 mg/kg/day by oral gavage, an increased incidence of hepatocellular adenoma/carcinoma was observed at 200 mg/kg/day at systemic exposures 20 times the human exposure at 40 mg/day based on AUC. An increased incidence of hepatocellular tumors was not seen at lower doses.
Rosuvastatin was not mutagenic or clastogenic with or without metabolic activation in the Ames test with Salmonella typhimurium and Escherichia coli, the mouse lymphoma assay, and the chromosomal aberration assay in Chinese hamster lung cells. Rosuvastatin was negative in the in vivo mouse micronucleus test.
In rat fertility studies with oral gavage doses of 5, 15, 50 mg/kg/day, males were treated for 9 weeks prior to and throughout mating and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 50 mg/kg/day (systemic exposures up to 10 times the human exposure at 40 mg/day based on AUC). In testicles of dogs treated with rosuvastatin at 30 mg/kg/day for one month, spermatidic giant cells were seen. Spermatidic giant cells were observed in monkeys after 6-month treatment at 30 mg/kg/day in addition to vacuolation of seminiferous tubular epithelium. Exposures in the dog were 20 times and in the monkey 10 times the human exposure at 40 mg/day based on body surface area. Similar findings have been seen with other drugs in this class.
13.2 Animal Toxicology and/or Pharmacology
Central Nervous System Toxicity
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with several other members of this drug class. A chemically similar drug in this class produced dose-dependent optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in dogs, at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose. Edema, hemorrhage, and partial necrosis in the interstitium of the choroid plexus was observed in a female dog sacrificed moribund at day 24 at 90 mg/kg/day by oral gavage (systemic exposures 100 times the human exposure at 40 mg/day based on AUC). Corneal opacity was seen in dogs treated for 52 weeks at 6 mg/kg/day by oral gavage (systemic exposures 20 times the human exposure at 40 mg/day based on AUC). Cataracts were seen in dogs treated for 12 weeks by oral gavage at 30 mg/kg/day (systemic exposures 60 times the human exposure at 40 mg/day based on AUC). Retinal dysplasia and retinal loss were seen in dogs treated for 4 weeks by oral gavage at 90 mg/kg/day (systemic exposures 100 times the human exposure at 40 mg/day based on AUC). Doses ≤ 30 mg/kg/day (systemic exposures ≤ 60 times the human exposure at 40 mg/day based on AUC) did not reveal retinal findings during treatment for up to one year.
Juvenile Toxicology Study
In a juvenile study, rats were dosed by oral gavage with 10 or 50 mg/kg/day from weaning for 9 weeks prior to pairing, throughout pairing and up to the day before necropsy for males or up to gestation day 7 for females. No effects on sexual development, testicular and epididymal appearance or fertility were observed at either dose level.
Pediatric information is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
14 CLINICAL STUDIES
14.1 Hyperlipidemia and Mixed Dyslipidemia
Rosuvastatin reduces Total-C, LDL-C, ApoB, nonHDL-C, and TG, and increases HDL-C, in adult patients with hyperlipidemia and mixed dyslipidemia.
Dose-Ranging Study: In a multicenter, double-blind, placebo-controlled, dose-ranging study in patients with hyperlipidemia rosuvastatin given as a single daily dose for 6 weeks significantly reduced Total-C, LDL-C, nonHDL-C, and ApoB, across the dose range (Table 6).
|
Dose |
N |
Total-C |
LDL-C |
Non-HDL-C |
ApoB |
TG |
HDL-C |
|
Placebo |
13 |
-5 |
-7 |
-7 |
-3 |
-3 |
3 |
|
Rosuvastatin 5 mg |
17 |
-33 |
-45 |
-44 |
-38 |
-35 |
13 |
|
Rosuvastatin 10 mg |
17 |
-36 |
-52 |
-48 |
-42 |
-10 |
14 |
|
Rosuvastatin 20 mg |
17 |
-40 |
-55 |
-51 |
-46 |
-23 |
8 |
|
Rosuvastatin 40 mg |
18 |
-46 |
-63 |
-60 |
-54 |
-28 |
10 |
Active-Controlled Study: Rosuvastatin was compared with the HMG-CoA reductase inhibitors atorvastatin, simvastatin, and pravastatin in a multicenter, open-label, dose-ranging study of 2240 patients with hyperlipidemia or mixed dyslipidemia. After randomization, patients were treated for 6 weeks with a single daily dose of either rosuvastatin, atorvastatin, simvastatin, or pravastatin (Figure 1 and Table 7).
Figure 1. Percent LDL-C Change by Dose of Rosuvastatin, Atorvastatin, Simvastatin, and Pravastatin at Week 6 in Patients with Hyperlipidemia or Mixed Dyslipidemia
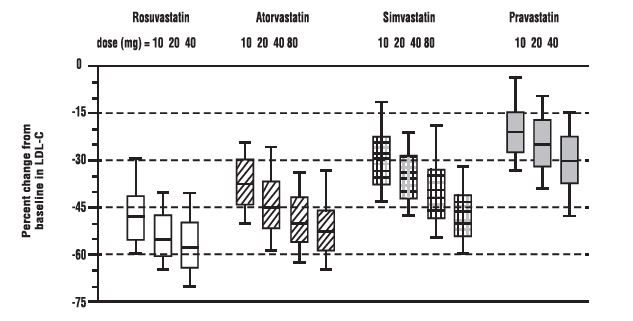
Box plots are a representation of the 25th, 50th, and 75th percentile values, with whiskers representing the 10th and 90th percentile values. Mean baseline LDL-C: 189 mg/dL
|
||||
|
Treatment Daily Dose |
||||
|
Treatment |
10 mg |
20 mg |
40 mg |
80 mg |
|
Rosuvastatin |
-46 † |
-52 ‡ |
-55 § |
--- |
|
Atorvastatin |
-37 |
-43 |
-48 |
-51 |
|
Simvastatin |
-28 |
-35 |
-39 |
-46 |
|
Pravastatin --- |
-20 |
-24 |
-30 | |
14.2 Heterozygous Familial Hypercholesterolemia
Active-Controlled Study: In a study of patients with heterozygous FH (baseline mean LDL of 291), patients were randomized to rosuvastatin 20 mg or atorvastatin 20 mg. The dose was increased by 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (Table 8).
|
|||
|
Rosuvastatin (n=435) LS Mean* (95%CI) |
Atorvastatin(n=187) LS Mean* (95% CI) |
||
|
Week 6 |
20 mg |
-47% (-49%, -46%) |
-38% (-40%, -36%) |
|
Week 12 |
40 mg |
-55% (-57%, -54%) |
-47% (-49%, -45%) |
|
Week 18 |
80 mg |
NA |
-52% (-54%, -50%) |
14.3 Hypertriglyceridemia
Dose-Response Study: In a double-blind, placebo-controlled dose-response study in patients with baseline TG levels from 273 to 817 mg/dL, rosuvastatin calcium given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 9).
|
Dose |
Placebo (n=26) |
Rosuvastatin 5 mg (n=25) |
Rosuvastatin 10 mg (n=23) |
Rosuvastatin 20 mg (n=27) |
Rosuvastatin 40 mg (n=25) |
|
Triglycerides |
1 (-40, 72) |
-21 (-58, 38) |
-37 (-65, 5) |
-37 (-72, 11) |
-43 (-80, -7) |
|
non-HDL-C |
2 (-13, 19) |
-29 (-43, -8) |
-49 (-59, -20) |
-43 (-74, 12) |
-51 (-62, -6) |
|
VLDL-C |
2 (-36, 53) |
-25 (-62, 49) |
-48 (-72, 14) |
-49 (-83, 20) |
-56 (-83, 10) |
|
Total-C |
1 (-13, 17) |
-24 (-40, -4) |
-40 (-51, -14) |
-34 (-61, -11) |
-40 (-51, -4) |
|
LDL-C |
5 (-30, 52) |
-28 (-71, 2) |
-45 (-59, 7) |
-31 (-66, 34) |
-43 (-61, -3) |
|
HDL-C |
-3 (-25, 18) |
3 (-38, 33) |
8 (-8, 24) |
22 (-5, 50) |
17 (-14, 63) |
14.4 Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia)
In a randomized, multicenter, double-blind crossover study, 32 patients (27 with ε2/ε2 and 4 with apo E mutation [Arg145Cys] with primary dysbetalipoproteinemia (Type III Hyperlipoproteinemia) entered a 6-week dietary lead-in period on the NCEP Therapeutic Lifestyle Change (TLC) diet. Following dietary lead-in, patients were randomized to a sequence of treatments in conjunction with the TLC diet for 6 weeks each: rosuvastatin 10 mg followed by rosuvastatin 20 mg or rosuvastatin 20 mg followed by rosuvastatin 10 mg. Rosuvastatin reduced non HDL-C (primary end point) and circulating remnant lipoprotein levels. Results are shown in the table below.
|
Median at Baseline (mg/dL) |
Median percent change from baseline (95% CI) Rosuvastatin 10 mg |
Median percent change from baseline (95% CI) Rosuvastatin 20 mg |
|
|
Total-C |
342.5 |
-43.3 (-46.9, -37.5) |
-47.6 (-51.6, -42.8) |
|
Triglycerides |
503.5 |
-40.1 (-44.9, -33.6) |
-43.0 (-52.5, -33.1) |
|
Non-HDL-C |
294.5 |
-48.2 (-56.7, -45.6) |
-56.4 (-61.4, -48.5) |
|
VLDL-C + IDL-C |
209.5 |
-46.8 (-53.7, -39.4) |
-56.2 (-67.7, -43.7) |
|
LDL-C |
112.5 |
-54.4 (-59.1, -47.3) |
-57.3 (-59.4, -52.1) |
|
HDL-C |
35.5 |
10.2 (1.9, 12.3) |
11.2 (8.3, 20.5) |
|
RLP-C |
82.0 |
-56.4 (-67.1, -49.0) |
-64.9 (-74.0, -56.6) |
|
Apo-E |
16.0 |
-42.9 (-46.3, -33.3) |
-42.5 (-47.1, -35.6) |
14.5 Homozygous Familial Hypercholesterolemia
Dose-Titration Study: In an open-label, forced-titration study, homozygous FH patients (n=40, 8 to 63 years) were evaluated for their response to rosuvastatin 20 to 40 mg titrated at a 6-week interval. In the overall population, the mean LDL-C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL-C, the mean LDL-C reduction was 30% (median 28% reduction). Among 13 patients with an LDL-C reduction of <15%, 3 had no change or an increase in LDL-C. Reductions in LDL-C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
14.7 Pediatric Patients with Heterozygous Familial Hypercholesterolemia
In a double-blind, randomized, multicenter, placebo-controlled, 12-week study, 176 (97 male and 79 female) children and adolescents with heterozygous familial hypercholesterolemia were randomized to rosuvastatin 5, 10 or 20 mg or placebo daily. Patients ranged in age from 10 to 17 years (median age of 14 years) with approximately 30% of the patients
10 to 13 years and approximately 17%, 18%, 40%, and 25% at Tanner stages II, III, IV, and V, respectively. Females were at least 1 year postmenarche. Mean LDL-C at baseline was 233 mg/dL (range of 129 to 399). The 12-week double-blind phase was followed by a 40 week open label dose-titration phase, where all patients (n=173) received 5 mg, 10 mg or 20 mg rosuvastatin daily.
Rosuvastatin significantly reduced LDL-C (primary end point), total cholesterol and ApoB levels at each dose compared to placebo. Results are shown in Table 12 below.
|
||||||
|
Dose (mg) |
N |
LDL-C |
HDL-C |
Total-C |
TG |
ApoB |
|
Placebo |
46 |
-1% |
+7% |
0% |
-7% |
-2% |
|
5 |
42 |
-38% |
+4% * |
-30% |
-13% * |
-32% |
|
10 |
44 |
-45% |
+11% * |
-34% |
-15% * |
-38% |
|
20 |
44 |
-50% |
+9% * |
-39% |
16% * |
-41% |
At the end of the 12-week, double-blind treatment period, the percentage of patients achieving the LDL-C goal of less than 110 mg/dL (2.8 mmol/L) was 0% for placebo, 12% for rosuvastatin 5 mg, 41% for rosuvastatin 10 mg and 41% for rosuvastatin 20 mg. For the 40-week, open-label phase, 71% of the patients were titrated to the maximum dose of 20 mg and 41% of the patients achieved the LDL-C goal of 110 mg/dL.
Rosuvastatin was also studied in a two year open-label, uncontrolled, titration to goal trial that included 175 children and adolescents with heterozygous familial hypercholesterolemia who were 8 to 17 years old (79 boys and 96 girls). All patients had a documented genetic defect in the LDL receptor or in ApoB. Approximately 89% were Caucasian, 7% were Asian, 1% were Black, and fewer than 1% were Hispanic. Mean LDL-C at baseline was 236 mg/dL. Fifty-eight (33%) patients were prepubertal at baseline. The starting rosuvastatin dosage for all children and adolescents was 5 mg once daily. Children 8 to less than 10 years of age (n=41 at baseline) could titrate to a maximum dosage of 10 mg once daily, and children and adolescents 10 to 17 years of age could titrate to a maximum dosage of 20 mg once daily.
The reductions in LDL-C from baseline were generally consistent across age groups within the trial as well as with previous experience in both adult and pediatric controlled trials.
The long-term efficacy of rosuvastatin therapy initiated in childhood to reduce morbidity and mortality in adulthood has not been established.
14.8 Slowing of the Progression of Atherosclerosis
In the Measuring Effects on Intima Media Thickness: an Evaluation Of Rosuvastatin 40 mg study, the effect of therapy with rosuvastatin on carotid atherosclerosis was assessed by B-mode ultrasonography in patients with elevated LDL-C, at low risk (Framingham risk <10% over ten years) for symptomatic coronary artery disease and with subclinical atherosclerosis as evidenced by carotid intimal-medial thickness (cIMT). In this double-blind, placebo-controlled clinical study 984 patients were randomized (of whom 876 were analyzed) in a 5:2 ratio to rosuvastatin 40 mg or placebo once daily. Ultrasonograms of the carotid walls were used to determine the annualized rate of change per patient from baseline to two years in mean maximum cIMT of 12 measured segments. The estimated difference in the rate of change in the maximum cIMT analyzed over all 12 carotid artery sites between patients treated with rosuvastatin and placebo-treated patients was -0.0145 mm/year (95% CI –0.0196, –0.0093; p<0.0001).
The annualized rate of change from baseline for the placebo group was +0.0131 mm/year (p<0.0001). The annualized rate of change from baseline for the group treated with rosuvastatin was -0.0014 mm/year (p=0.32).
At an individual patient level in the group treated with rosuvastatin 52.1% of patients demonstrated an absence of disease progression (defined as a negative annualized rate of change), compared to 37.7% of patients in the placebo group.
14.9 Primary Prevention of Cardiovascular Disease
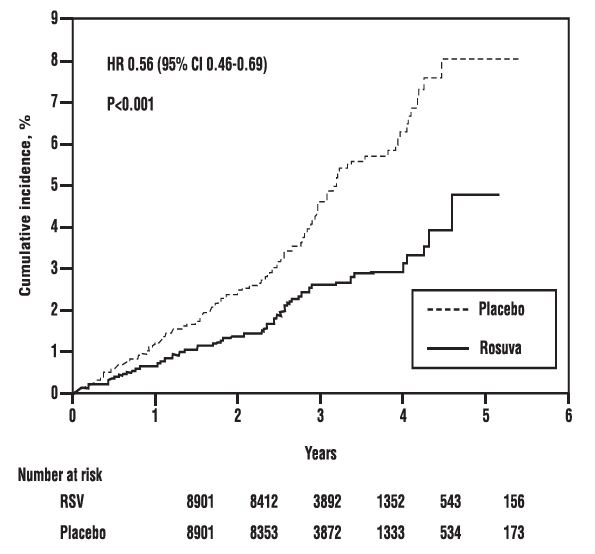
In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin study, the effect of rosuvastatin on the occurrence of major cardiovascular (CV) disease events was assessed in 17,802 men (≥50 years) and women (≥60 years) who had no clinically evident cardiovascular disease, LDL-C levels <130 mg/dL (3.3 mmol/l) and hs-CRP levels ≥2 mg/L. The study population had an estimated baseline coronary heart disease risk of 11.6% over 10 years based on the Framingham risk criteria and included a high percentage of patients with additional risk factors such as hypertension (58%), low HDL-C levels (23%), cigarette smoking (16%), or a family history of premature CHD (12%). Study participants had a median baseline LDL-C of 108 mg/dL and hsCRP of 4.3 mg/L. Study participants were randomly assigned to placebo (n=8901) or rosuvastatin 20 mg once daily (n=8901) and were followed for a mean duration of 2 years. The study was stopped early by the Data Safety Monitoring Board due to meeting predefined stopping rules for efficacy in rosuvastatin-treated subjects.
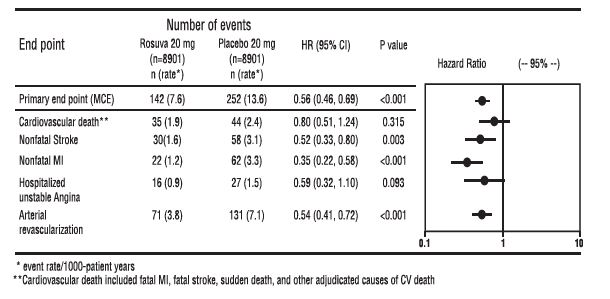
The primary end point was a composite end point consisting of the time-to-first occurrence of any of the following major CV events: CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina or an arterial revascularization procedure.
Rosuvastatin significantly reduced the risk of major CV events (252 events in the placebo group vs. 142 events in the rosuvastatin group) with a statistically significant (p<0.001) relative risk reduction of 44% and absolute risk reduction of 1.2% (see Figure 2). The risk reduction for the primary end point was consistent across the following predefined subgroups: age, sex, race, smoking status, family history of premature CHD, body mass index, LDL-C, HDL-C, and hsCRP levels.
Figure 2. Time to First Occurrence of Major Cardiovascular Events in Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin study
The individual components of the primary end point are presented in Figure 3. Rosuvastatin significantly reduced the risk of nonfatal myocardial infarction, nonfatal stroke, and arterial revascularization procedures. There were no significant treatment differences between the rosuvastatin and placebo groups for death due to cardiovascular causes or hospitalizations for unstable angina.
Rosuvastatin significantly reduced the risk of myocardial infarction (6 fatal events and 62 nonfatal events in placebo-treated subjects vs. 9 fatal events and 22 nonfatal events in rosuvastatin-treated subjects) and the risk of stroke (6 fatal events and 58 nonfatal events in placebo-treated subjects vs. 3 fatal events and 30 nonfatal events in rosuvastatin-treated subjects).
In a post-hoc subgroup analysis of Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin study subjects (n=1405; rosuvastatin=725, placebo=680) with a hsCRP ≥2 mg/L and no other traditional risk factors (smoking, BP ≥140/90 or taking antihypertensives, low HDL-C) other than age, after adjustment for high HDL-C, there was no significant treatment benefit with rosuvastatin treatment.
Figure 3. Major CV Events by Treatment Group in Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin study
At one year, rosuvastatin increased HDL-C and reduced LDL-C, hsCRP, total cholesterol and serum triglyceride levels (p<0.001 for all versus placebo).
16 HOW SUPPLIED/STORAGE AND HANDLING
Rosuvastatin Tablets USP 5 mg are pink, oval shaped, biconvex film-coated tablets debossed with ‘I’ on one side and ‘29’ on the other side.
Unit dose packages of 100 (10 x 10) NDC 60687-234-01
Rosuvastatin Tablets USP 10 mg are pink, round, biconvex film-coated tablets debossed with ‘I’ on one side and ‘30’ on the other side.
Unit dose packages of 50 (5 x 10) NDC 60687-245-65
Unit dose packages of 100 (10 x 10) NDC 60687-245-01
Rosuvastatin Tablets USP 20 mg are pink, round, biconvex film-coated tablets debossed with ‘I’ on one side and ‘31’ on the other side.
Unit dose packages of 100 (10 x 10) NDC 60687-256-01
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients should be instructed not to take 2 doses of rosuvastatin within 12 hours of each other.
Skeletal Muscle Effects
Patients should be advised to report promptly unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing rosuvastatin.
Concomitant Use of Antacids
When taking rosuvastatin with an aluminum and magnesium hydroxide combination antacid, the antacid should be taken at least 2 hours after rosuvastatin administration.
Embryofetal Toxicity
Advise females of reproductive potential of the risk to a fetus, to use effective contraception during treatment, and to inform their healthcare provider of a known or suspected pregnancy.
[see
Contraindications (4) and
Use in Specific Populations (8.1,
8.3)].
Lactation
Advise women not to breastfeed during treatment with rosuvastatin
[see
Contraindications (4) and
Use in Specific Populations (8.2)].
Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation of rosuvastatin and if signs or symptoms of liver injury occur. All patients treated with rosuvastatin should be advised to promptly report any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
CRESTOR is a trademark of the AstraZeneca group of companies.
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see
How Supplied section) contain drug product from Rising Health, LLC as follows:
(5 mg / 100 UD) NDC 60687-234-01 packaged from NDC 57237-168
(10 mg / 50 UD) NDC 60687-245-65 packaged from NDC 57237‐169
(10 mg / 100 UD) NDC 60687-245-01 packaged from NDC 57237-169
(20 mg / 100 UD) NDC 60687-256-01 packaged from NDC 57237-170
Distributed by:
American Health Packaging
Columbus, OH 43217
8423401/0523F
PATIENT INFORMATION
8423401/0523F
Rosuvastatin Tablets USP
(roe-SOO-va-STAT-in)
Read this Patient Information carefully before you start taking rosuvastatin tablets and each time you get a refill. If you have any questions about rosuvastatin tablets, ask your doctor. Only your doctor can determine if rosuvastatin tablets are right for you.
What are rosuvastatin tablets?
Rosuvastatin tablets are a prescription medicine that contains a cholesterol-lowering medicine called rosuvastatin calcium.
- Rosuvastatin tablets are used along with diet to:
- lower the level of your “bad” cholesterol (LDL)
- increase the level of your “good” cholesterol (HDL)
- lower the level of fat in your blood (triglycerides)
- slow the buildup of fatty deposits (plaque) in the walls of blood vessels
- Rosuvastatin tablets are used to treat:
- adults who cannot control their cholesterol levels by diet and exercise alone
- children 8 to 17 years of age with heterozygous familial hypercholesterolemia (an inherited condition that causes high levels of LDL)
Rosuvastatin tablets are not approved for use in children with heterozygous familial hypercholesterolemia younger than 8 years of age or for use in children with homozygous familial hypercholesterolemia younger than 7 years of age.
Rosuvastatin tablets are used to reduce the risk of heart attacks and strokes in men 50 years of age and older and women 60 years of age and older who do not have known heart disease but do have certain additional risk factors.
It is not known if rosuvastatin tablets are safe and effective in people who have Fredrickson Type I and V dyslipidemias.
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s CRESTOR (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Who should not take rosuvastatin tablets?
Do not take rosuvastatin tablets if you:
- are allergic to rosuvastatin calcium or any of the ingredients in rosuvastatin tablets. See the end of this leaflet for a complete list of ingredients in rosuvastatin tablets.
- have liver problems.
- are pregnant or think you may be pregnant, or are planning to become pregnant. Rosuvastatin tablets may harm your unborn baby. If you become pregnant, stop taking rosuvastatin tablets and call your doctor right away. If you are not planning to become pregnant you should use effective birth control (contraception) while you are taking rosuvastatin tablets.
- are breastfeeding. Medicines like rosuvastatin calcium can pass into your breast milk and may harm your baby.
What should I tell my doctor before and while taking rosuvastatin tablets?
Tell your doctor if you:
- have unexplained muscle aches or weakness
- have or have had kidney problems
- have or have had liver problems
- drink more than 2 glasses of alcohol daily
- have thyroid problems
- are 65 years of age or older
- are of Asian descent
- are pregnant or think you may be pregnant, or are planning to become pregnant
- are breastfeeding
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Talk to your doctor before you start taking any new medicines.
Taking rosuvastatin tablets with certain other medicines may affect each other causing side effects. Rosuvastatin tablets may affect the way other medicines work, and other medicines may affect how rosuvastatin tablets work.
Especially tell your doctor if you take:
- cyclosporine (a medicine for your immune system)
- gemfibrozil (a fibric acid medicine for lowering cholesterol)
- darolutamide (a medicine for the treatment of prostate cancer)
- regorafenib (a medicine used to treat cancer of the colon and rectum)
- anti-viral medicines including certain HIV or hepatitis C virus drugs such as:
- lopinavir, ritonavir, fosamprenavir, tipranavir, atazanavir, simeprevir
- combination of
- sofosbuvir/velpatasvir/voxilaprevir
- dasabuvir/ombitasvir/paritaprevir/ritonavir
- elbasvir/grazoprevir
- sofosbuvir/velpatasvir
- glecaprevir/pibrentasvir and
- all other combinations with ledipasvir including ledipasvir/sofosbuvir
- certain anti-fungal medicines (such as itraconazole, ketoconazole and fluconazole)
- coumarin anticoagulants (medicines that prevent blood clots, such as warfarin)
- niacin or nicotinic acid
- fibric acid derivatives (such as fenofibrate)
- colchicine (a medicine used to treat gout)
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know all of the medicines you take. Keep a list of them to show your doctor and pharmacist when you get new medicine.
How should I take rosuvastatin tablets?
- Take rosuvastatin tablets exactly as your doctor tells you to take them.
- Take rosuvastatin tablets, by mouth, 1 time each day. Swallow the tablet whole.
- Rosuvastatin tablets can be taken at any time of day, with or without food.
- Do not change your dose or stop rosuvastatin tablets without talking to your doctor, even if you are feeling well.
- Your doctor may do blood tests to check your cholesterol levels before and during your treatment with rosuvastatin tablets. Your doctor may change your dose of rosuvastatin tablets if needed.
- Your doctor may start you on a cholesterol lowering diet before giving you rosuvastatin tablets. Stay on this diet when you take rosuvastatin tablets.
- Wait at least 2 hours after taking rosuvastatin tablets to take an antacid that contains a combination of aluminum and magnesium hydroxide.
- If you miss a dose of rosuvastatin tablets, take it as soon as you remember. However, do not take 2 doses of rosuvastatin tablets within 12 hours of each other.
- If you take too much rosuvastatin calcium or overdose, call your doctor or go to the nearest hospital emergency room right away.
What are the Possible Side Effects of Rosuvastatin Tablets?
Rosuvastatin tablets may cause serious side effects, including:
- Muscle pain, tenderness and weakness (myopathy). Muscle problems, including muscle breakdown, can be serious in some people and rarely cause kidney damage that can lead to death. Tell your doctor right away if:
- you have unexplained muscle pain, tenderness, or weakness, especially if you have a fever or feel more tired than usual, while you take rosuvastatin tablets.
- you have muscle problems that do not go away even after your doctor has told you to stop taking rosuvastatin tablets. Your doctor may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you:
- are taking certain other medicines while you take rosuvastatin tablets
- are 65 years of age or older
- have thyroid problems (hypothyroidism) that are not controlled
- have kidney problems
- are taking higher doses of rosuvastatin tablets
- Liver problems. Your doctor should do blood tests to check your liver before you start taking rosuvastatin tablets and if you have symptoms of liver problems while you take rosuvastatin tablets. Call your doctor right away if you have any of the following symptoms of liver problems:
- feel unusually tired or weak
- loss of appetite
- upper belly pain
- dark urine
- yellowing of your skin or the whites of your eyes
The most common side effects may include: headache, muscle aches and pains, abdominal pain, weakness, and nausea.
Additional side effects that have been reported with rosuvastatin tablets include memory loss and confusion.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of rosuvastatin tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store rosuvastatin tablets?
- Store rosuvastatin tablets at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature] and in a dry place.
- Safely throw away medicine that is out of date or no longer needed.
Keep rosuvastatin tablets and all medicines out of the reach of children.
What are the Ingredients in rosuvastatin tablets?
Active Ingredient: rosuvastatin as rosuvastatin calcium
Inactive Ingredients: crospovidone, dibasic calcium phosphate anhydrous, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
General Information about the safe and effective use of rosuvastatin tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use rosuvastatin tablets for a condition for which it was not prescribed. Do not give rosuvastatin tablets to other people, even if they have the same medical condition you have. They may harm them.
You can ask your pharmacist or doctor for information about rosuvastatin tablets that is written for health professionals.
CRESTOR is a trademark of the AstraZeneca group of companies.
For more information about the drug product, call Rising Health, LLC at 1-833-395-6928.
For more information about the packaging and labeling, call American Health Packaging at 1-800-707-4621.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
American Health Packaging
Columbus, OH 43217
8423401/0523F
Package/Label Display Panel – Carton – 5 mg

NDC 60687- 234-01
Rosuvastatin
Tablets USP
5 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Rosuvastatin calcium USP equivalent to rosuvastatin
5 mg.
Usual Dosage: See package insert for full prescribing
information.
Store at 20° to 25°C (68° to 77°F); excursions
permitted between 15° to 30°C (59° to 86°F) [see USP
Controlled Room Temperature]. Protect from
moisture.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is
torn or broken.
The drug product contained in this package is from
NDC # 57237-168, Rising Health, LLC.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
723401
0423401/0120OS
Package/Label Display Panel – Carton – 10 mg – 50 UD

NDC 60687- 245-65
Rosuvastatin
Tablets USP
10 mg
50 Tablets (5 x 10) Rx Only
Each Film-Coated Tablet Contains:
Rosuvastatin calcium USP equivalent to rosuvastatin 10 mg.
Usual Dosage: See package insert for full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from moisture.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 57237-169, Rising Health, LLC.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
724565
0424565/0523
Package/Label Display Panel – Carton – 10 mg – 100 UD

NDC 60687- 245-01
Rosuvastatin
Tablets USP
10 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Rosuvastatin calcium USP equivalent to rosuvastatin
10 mg.
Usual Dosage: See package insert for full prescribing
information.
Store at 20° to 25°C (68° to 77°F); excursions
permitted between 15° to 30°C (59° to 86°F) [see USP
Controlled Room Temperature]. Protect from moisture.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn
or broken.
The drug product contained in this package is from
NDC # 57237-169, Rising Health, LLC.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
724501
0424501/1219OS
Package/Label Display Panel – Carton – 20 mg

NDC 60687- 256-01
Rosuvastatin
Tablets USP
20 mg
100 Tablets (10 x 10) Rx Only
Each Film-Coated Tablet Contains:
Rosuvastatin calcium USP equivalent to rosuvastatin
20 mg.
Usual Dosage: See package insert for full prescribing
information.
Store at 20° to 25°C (68° to 77°F); excursions
permitted between 15° to 30°C (59° to 86°F) [see USP
Controlled Room Temperature]. Protect from moisture.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn
or broken.
The drug product contained in this package is from
NDC # 57237-170, Rising Health, LLC.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
725601
0425601/1019OS