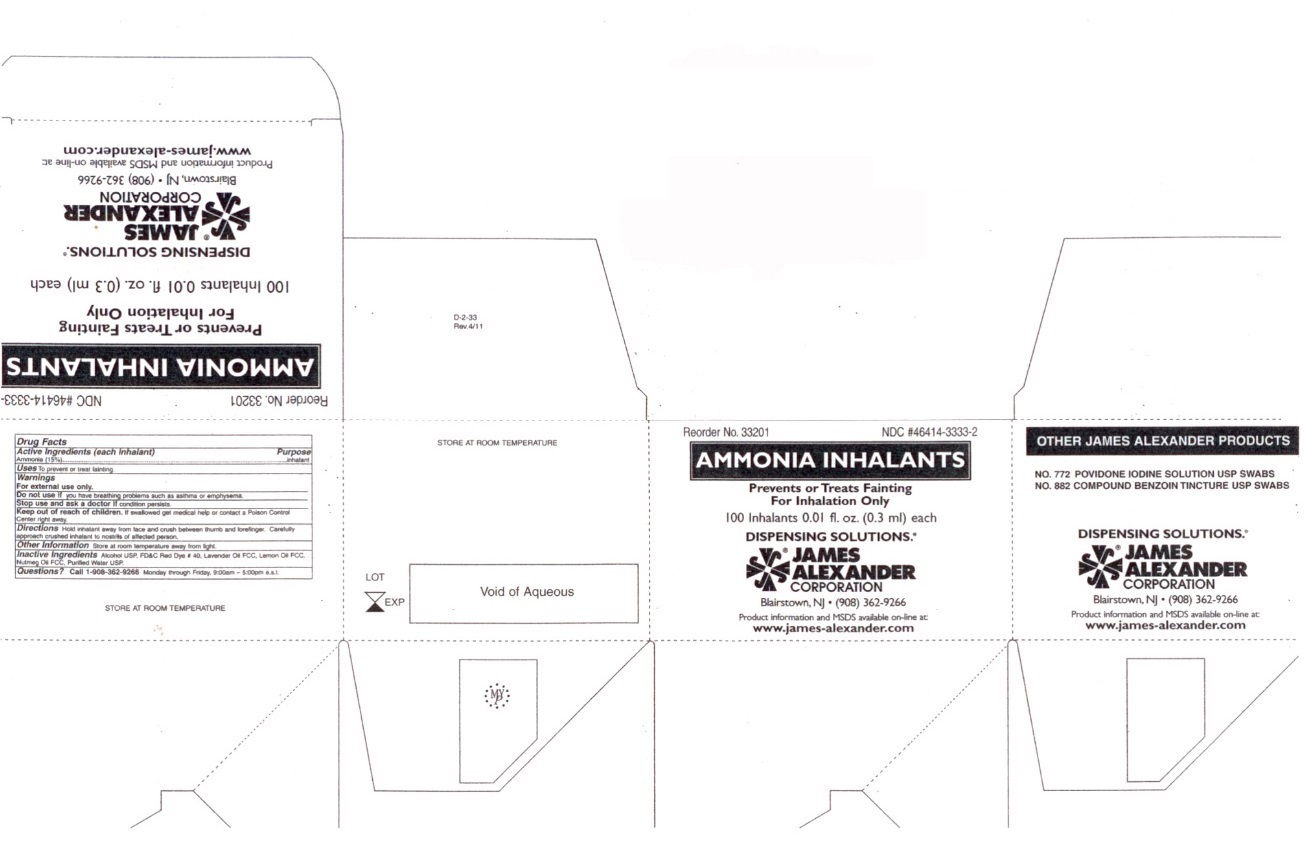
Warnings
For external use only.
Do not use if you have breathing problems such as asthma or emphysema.
Stop use and ask a doctor if condition persists.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away.
Directions
Hold inhalant away from face and crush between thumb and forefinger. Carefully approach crushed inhalant to nostrils of affected person.
Inactive Ingredients
Alcohol USP, FD&C Red Dye # 40, Lavender Oil FCC, Lemon Oil FCC, Nutmeg Oil FCC, Purified Water USP.