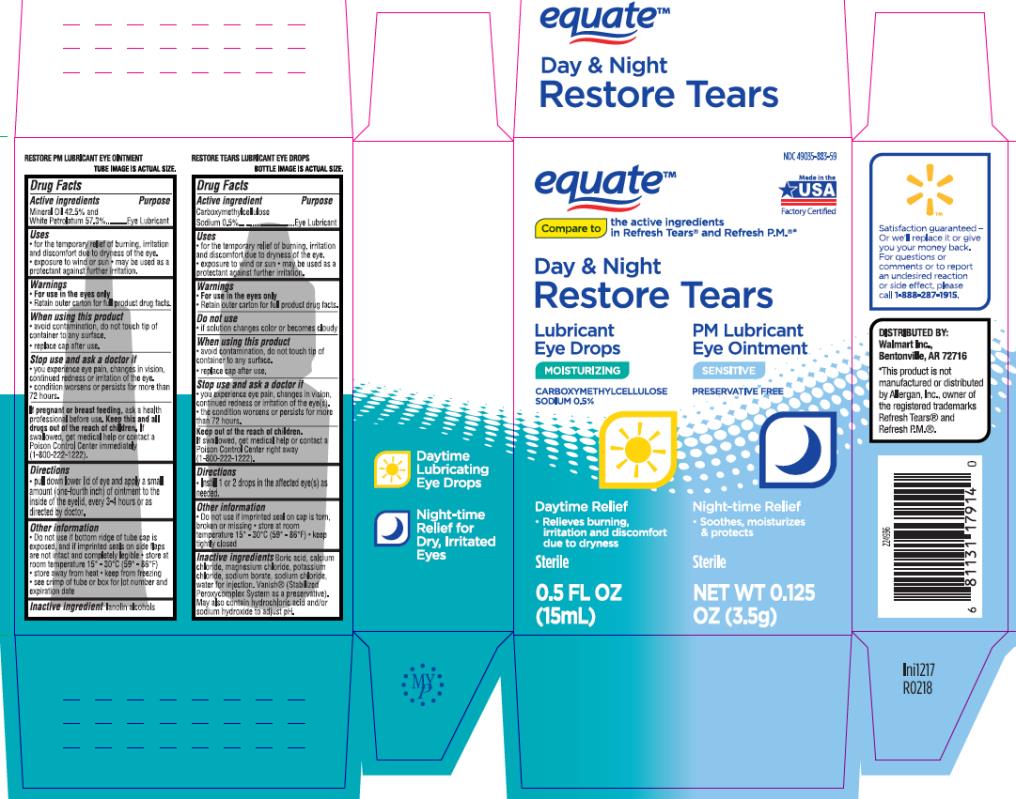
Walmart
Equate
Day & Night Restore Tears
15mL & 3.5g
NDC 49035-883-59
Restore PM Lubricant Eye Ointment NDC# 49035-191-50
Drug Facts
Uses
- for the temporary relief of burning, irritation and discomfort due to dryness of the eye.
- exposure to wind or sun
- may be used as a protectant against further irritation.
Warnings
-
For use in the eyes only.
- Retain outer carton for full product drug facts.
When using this product
- avoid contamination, do not touch tip of container to any surface.
- replace cap after use.
Directions
- pull down lower lid of eye and apply a small amount (one-fourth inch) of ointment to the inside of the eyelid, every 3-4 hours or as directed by doctor.
Other information
- Do not use if bottom ridge of tube cap is exposed, and if imprinted seals on side flaps are not intact and completely legible • store at room temperature 15°-30°C (59°-86°F)
- store away from heat
- keep from freezing
- see crimp of tube or box for lot number and expiration date
Inactive ingredients
lanolin alcohols
Walmart
Equate
Day & Night Restore Tears
15mL & 3.5g
NDC 49035-883-59
Restore Tears Lubricant Eye Drops NDC 49035-189-13
Drug Facts
Uses
- for the temporary relief of burning, irritation and discomfort due to dryness of the eye.
- exposure to wind or sun
- may be used as a protectant against further irritation.
Warnings
-
For use in the eyes only.
- Retain outer carton for full product drug facts.
When using this product
- avoid contamination, do not touch tip of container to any surface.
- replace cap after use.
Other information
- Do not use if imprinted seal on cap is torn, broken or missing
- store at room temperature 15°-30°C (59°-86°F)
- keep tightly closed