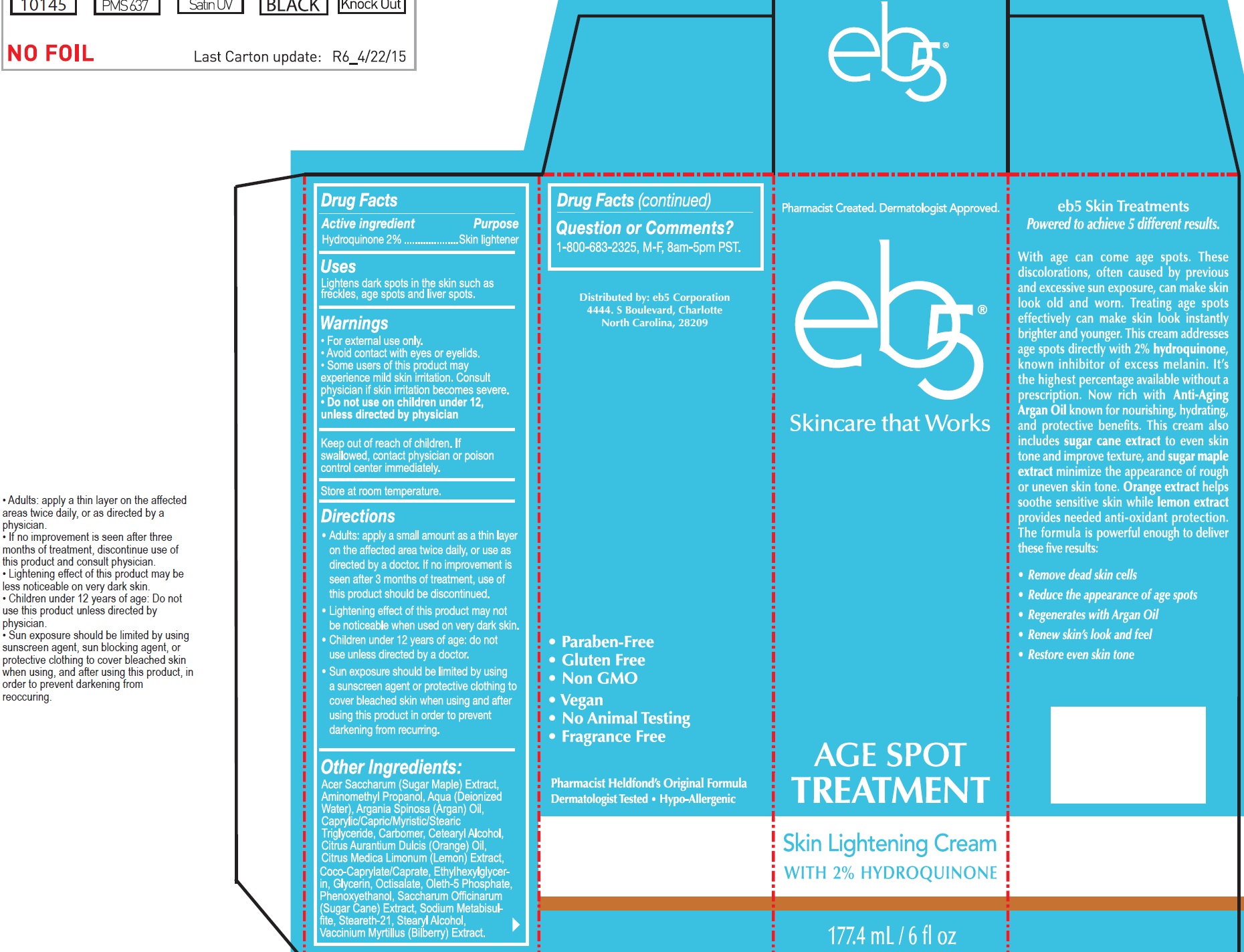
Active ingredient
Hydroquinone 2%
Uses
Lightens dark spots in the skin such as freckles, age spots an liver spots.
Warnings
- For external use only.
- Avoid contact with eyes or eyelids.
- Some users of this product may experience milld skin irritation. Consult physician if skin irritation becomes severe.
Do not use
- on children under 12, unless directed by physician
Keep out of reach of children.
If swallowed, contact physician or poison control center immediately.
Store at room temperature.
Directions
- Adults: apply a small amount as a thin layer on the affected area twice daily, or use as directed by a doctor. If no improvement is seen after 3 months of treatment, use of this product should be discontinued.
- Lightening effect of this product may not be noticeable when used on very dark skin.
- Children under 12 years of age: do not use unless directed by a doctor.
- Sun exposure should be limited by using a sunscreen agent or protective clothing to cover bleached skin when using and after using this product in order to prevent darkening from recurring.
Other Ingredients:
Acer Saccharum (Sugar Maple) Extract, Aminomethyl Propanol, Aqua (Deionized Water), Argania Spinosa (Argan) Oil, Caprylic/Capric/Myristic/Stearic Triglyceride, Carbomer, Cetearyl Alcohol, Citrus Aurantium Dulcis (Orange) Oil, Citrus Medica Limonum (Lemon) Extract, Coco-Caprylate/Caprate, Ethylhexylglycerin, Glycerin, Octisalate, Oleth-5 Phosphate, Phenoxyethanol, Saccharum Officinarum (Sugar Cane) Extract, Sodium Metabisulfite, Steareth-21, Stearyl Alcohol, Vaccinium Myrtillus (Bilberry) Extract.
Question of Comments?
1-800-683-2325, M-F, 8am-5pm PST.
Package Labeling:
Elements Brands, Inc.
