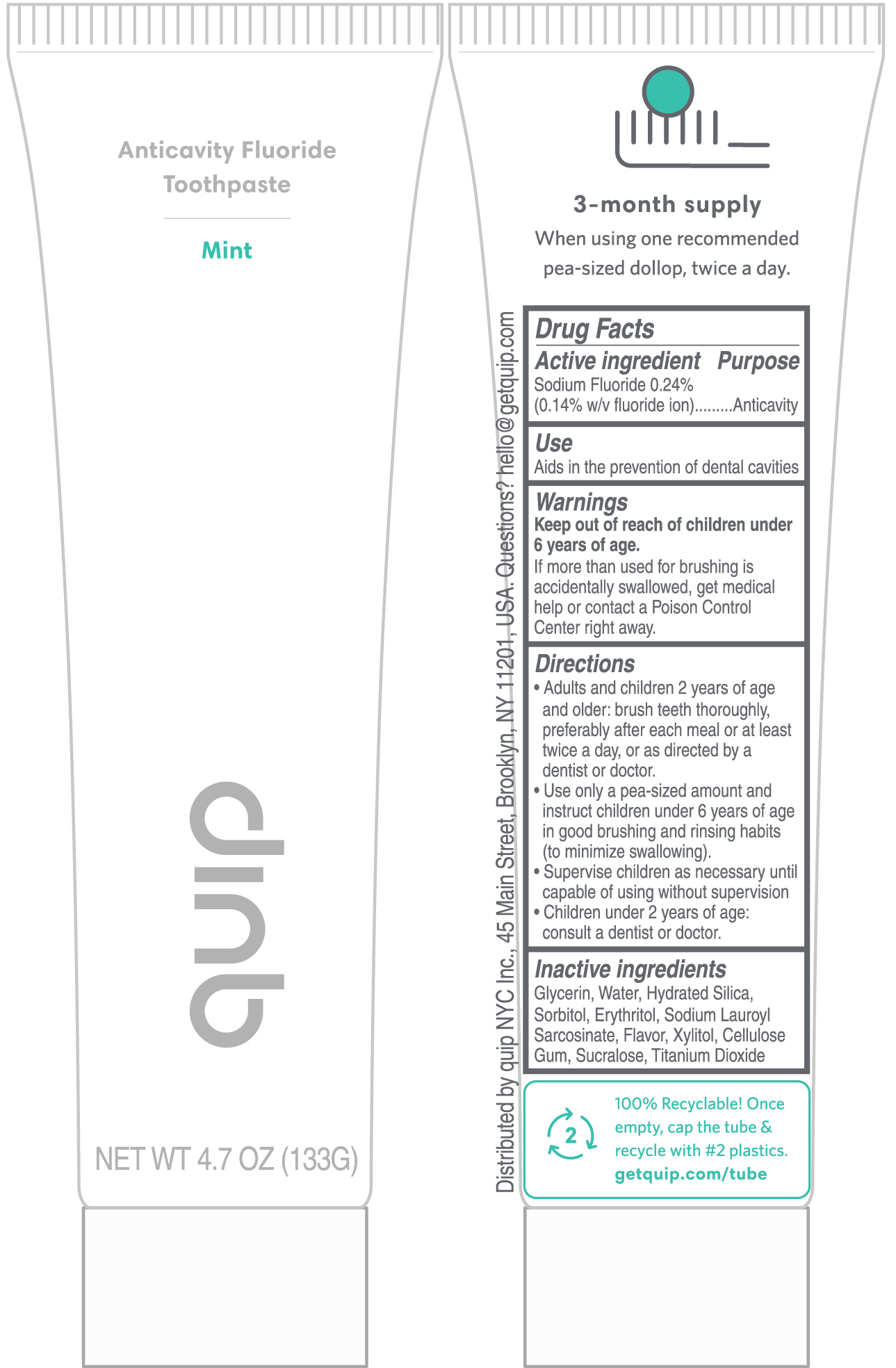
Keep out of reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Use only a pea-sized amount and instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision
- Children under 2 years of age: consult a dentist or doctor.
Inactive ingredients
Glycerin, Water, Hydrated Silica, Sorbitol, Erythritol, Sodium Lauroyl Sarcosinate, Flavor, Xylitol, Cellulose Gum, Sucralose, Titanium Dioxide