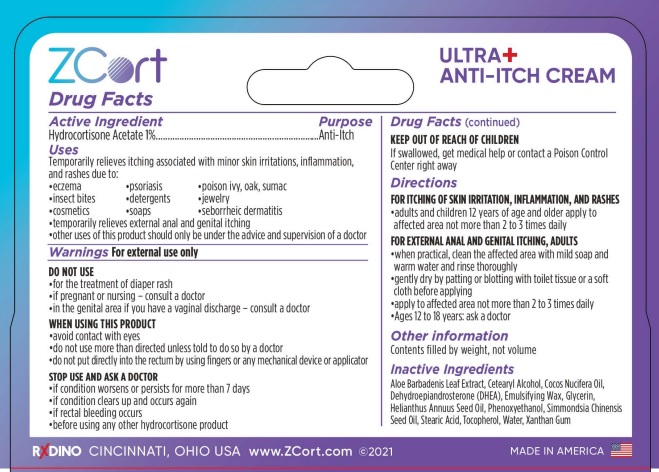
Uses
Temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- Eczema
- psoriasis
- poison ivy, oak, sumac
- insect bites
- detergents
- jewelry
- cosmetics
- soaps
- seborrheic dermatitis
- temporarily relieves external anal and genital itching
- other uses of this product should only be under the advice and supervision of a doctor
DO NOT USE
- for the treatment of diaper rash
- if pregnant or nursing - consult a doctor
- in the genital area if you have a vaginal discharge - consult a doctor
WHEN USING THIS PRODUCT
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
- do not put directly into the rectum by using fingers or any mechanical device or applicator
STOP USE AND ASK A DOCTOR
- if condition worsens or persists for more than 7 days
- if condition clears up and occurs again
- if rectal bleeding occurs
- before using any other hydrocortisone product
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away
Directions
FOR ITCHING OF SKIN IRRITATION, INFLAMMATION, AND RASHES
- adults and children 12 years of age and older apply to affected area not more than 2 to 3 times daily
FOR EXTERNAL ANAL AND GENITAL ITCHING, ADULTS
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 2 to 3 times daily
- Ages 12 to 18 years: ask a doctor
Inactive Ingredients
Aloe Barbadenis Leaf Extract, Cetearyl Alcohol, Cocos Nucifera Oil, Dehydroepiandrosterone (DHEA), Emulsifying Wax, Glycerin, Helianthus Annuus Seed Oil, Phenoxyethanol, Simmondsia Chinensis Seed Oil, Stearic Acid, Tocopherol, Water, Xanthan Gum
Front Display Panel and Back Card
NEW!
FORMULATED TO HEAL ITCH AND PROTECT COLLAGEN
STEROID POWER
FAST ACTING
- Rash
- Poison Ivy
- Eczema
- Insect Bites
- Psoriasis
- Inflammation
UNIQUE PATENTED FORMULA
ZCort ULTRA+ ANTI-ITCH CREAM
NET WT 0.5 OZ (15g)
ACTIVE INGREDIENT: HYDROCORTIZONE 1% WITH DHEA
see zcortfront.jpg

ZCort Ultra+ ANTI-ITCH CREAM BACK CARD
see zcortbackbox.jpg