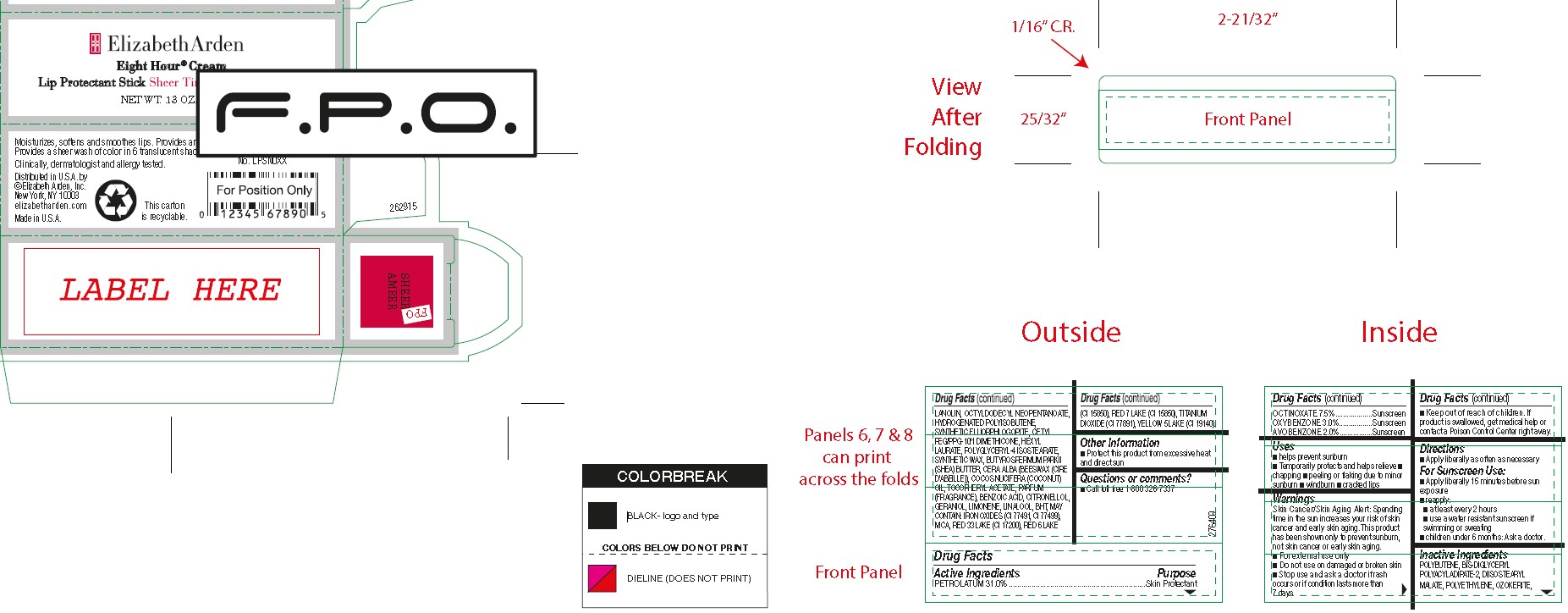
ACTIVE INGREDIENTS
OCTINOXATE 7.5%...................Sunscreen
OXYBENZONE 3.0%.................Sunscreen
AVOBENZONE 2.0%.................Sunscreen
INACTIVE INGREDIENTS
POLYBUTENE, BIS-DIGLYCERYL POLYACYLADIPATE-2, DIISOSTEARYL MALATE, POLYETHYLENE, OZOKERITE, LANOLIN, OCTYLDODECYL NEOPENTANOATE, HYDROGENATED POLYISOBUTENE, SYNTHETIC FLUORPHLOGOPITE, CETYL
PEG/PPG-10/1 DIMETHICONE, HEXYL LAURATE, POLYGLYCERYL-4 ISOSTEARATE, SYNTHETIC WAX, BUTYROSPERMUM PARKII (SHEA) BUTTER, CERA ALBA (BEESWAX (CIRE D’ABEILLE)), COCOS NUCIFERA (COCONUT) OIL, TOCOPHERYL ACETATE, PARFUM (FRAGRANCE), BENZOIC ACID, CITRONELLOL, GERANIOL, LIMONENE, LINALOOL, BHT,
MAY CONTAIN: IRON OXIDES (CI 77491, CI 77499), MICA, RED 33 LAKE (CI 17200), RED 6 LAKE (CI 15850), RED 7 LAKE (CI 15850), TITANIUM DIOXIDE (CI 77891), YELLOW 5 LAKE (CI 19140).
USES
■ helps prevent sunburn
■ Temporarily protects and helps relieve
■ chapping
■ peeling or flaking due to minor sunburn
■ windburn
■ cracked lips
WARNINGS
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
■ For external use only
■ Do not use on damaged or broken skin
■ Stop use and ask a doctor if rash occurs or
■ Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Directions
■ Apply liberally as often as necessary
For Sunscreen Use:
■ Apply liberally 15 minutes before sun exposure
■ reapply:
■ at least every 2 hours
■ use a water resistant sunscreen if swimming or sweating
■ children under 6 months: Ask a doctor.
- Helps prevent sunburn
- Temporarily protects and helps relieve
- peeling or flaking due to minor sunburn
- windburn
- cracked lips