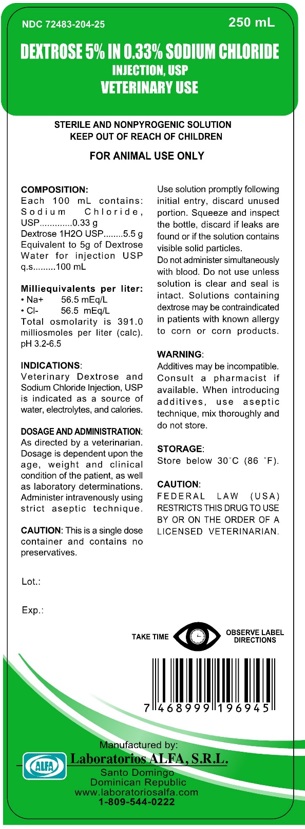
DESCRIPTION
Veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP is a sterile, non-pyrogenic solution for fluid replenishment in single dose containers for intravenous administration. Discard unused portion. It contains no antimicrobial agents.
|
Size (mL) | Composition (g/100 mL) | *Osmolarity (mOsmol/L) (Calculated) | pH | Concentration (mEq/L) | Caloric Content (kcal/L) | ||
| Dextrose 1H2O | Sodium Chloride, USP (NaCl) | Sodium | Chloride | ||||
| 100 | 5.5 | 0.33 | 391 | 3.2-6.5 | 56.5 | 56.5 | 170 |
| 250 | |||||||
| 500 | |||||||
| 1000 | |||||||
No venting is necessary during infusion.
CLINICAL PHARMACOLOGY
Veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP has value as a source of water, electrolytes. It is capable of inducing diuresis depending on the clinical condition of the patient.
INDICATIONS AND USAGE
Veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP is indicated as a source of water, electrolytes and calories.
CONTRAINDICATIONS
Solutions containing 5% Dextrose in 0.33% Sodium Chloride Injection, USP may be contraindicated in patients with known allergy to corn or corn products.
WARNINGS
Excessive administration of 5% Dextrose in 0.33% Sodium Chloride Injection, USP may result in significant hypokalemia.
Veterinary 5 % Dextrose in 0.33% Sodium Chloride Injection, USP, should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
Veterinary 5 % Dextrose in 0.33% Sodium Chloride Injection, USP should not be administered simultaneously with blood through the same administration set because of the possibility of pseudo agglutination or hemolysis.
The container label for these injections bears the statement: Do not administer simultaneously with blood.
The intravenous administration of veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, over-hydration, congested states, or pulmonary edema.
The risk of dilutive states is inversely proportional to the electrolyte concentration of the injections.
The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of the injections.
In patients with diminished renal function, administration of veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP may result in sodium retention proportional to the electrolyte concentrations of the injections.
Keep out of the reach of children.
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary
PRECAUTIONS
5% Dextrose in 0.33 Sodium Chloride Injection, USP should be used with caution in patients with overt or subclinical diabetes mellitus.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP to patients receiving corticosteroids or corticotrophin.
Veterinary 5% Dextrose in 0.33% Sodium Chloride Injection, USP should be used with caution in patients with known subclinical or overt diabetes mellitus.
Do not administer unless solution is clear and both seal and container are intact.
DOSAGE AND ADMINISTRATION
As directed by a veterinarian. Dosage is dependent upon the age, weight and clinical condition of the patient, as well as laboratory determinations.
Parenteral drug products should be inspected visually for particulate matter and discolorations prior to administration whenever solution and container permit.
All injections in plastic containers are intended for intravenous administration using sterile equipment.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. If, in the informed judgment of the veterinarian, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced.
Do not store solutions containing additives. Discard unused portion.
OVERDOSAGE
In an event of over hydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See Warnings, Precautions and Adverse Reactions.
PRECAUTION FOR USE OF THE BOTTLE
This is a single dose container and does not contain preservatives. If leaks are found, discard solution as sterility may be impaired.
Use the solution immediately after the bottle is opened, discard the remaining one. Discard unused portion. If supplemental medication is desired follow directions below:
Do not administer simultaneously with blood.
Do not use it unless solution is clear and seal is intact, the solution containing dextrose may be contraindicated in patients with a known allergy to corn or corn products.
Preparation and administration
- Check for minute leaks by squeezing the container firmly. If leaks are found, discard solution as sterility may be impaired.
- Suspend container from eyelet support.
- Remove Plastic protector from ports area at the bottom of container.
- Hold the bottle in vertical position and inset pyrogen free IV administration set in the outlet port. Use aseptic Technique
To add medication
WARNING: Additives may be incompatible.
To add medication before solution administration
1. Prepare medication site.
2. Using syringe with 19 to 22 gauge needle, puncture inlet port and inject.
3. Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To add medication during solution administration
1. Close clamp on the set.
2. Prepare medication site.
3. Using syringe with 18 to 21 gauge needle, puncture inlet port and inject.
4. Remove container from IV pole and/or turn to an upright position.
5. Mix solution and medication thoroughly.
6. Return container to in use position and continue administration.
CAUTION: Federal law (USA) restricts this drug to use by or on the order of a licensed veterinarian.
PRINCIPAL DISPLAY PANEL
Veterinary 5% Dextrose in 0.33% Sodium Chloride Injection,
USP Injection
VET DEXTROSE 5% IN 0.33% SODIUM CHLORIDE 1000 ML….NDC: 72483-204-10
VET DEXTROSE 5% IN 0.33% SODIUM CHLORIDE 500 ML…...NDC: 72483-204-05
VET DEXTROSE 5% IN 0.33% SODIUM CHLORIDE 250 ML…...NDC: 72483-204-25
VET DEXTROSE 5% IN 0.33% SODIUM CHLORIDE 100 ML…...NDC: 72483-204-01