Drug Facts
Active ingredient
Olopatadine (0.1%).
(equivalent to olopatadine hydrochloride, USP 0.111%)
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
Keep Out of Reach of Children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age:
consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate, hydrochloric acid/sodium hydroxide (adjust pH), sodium chloride and water for injection
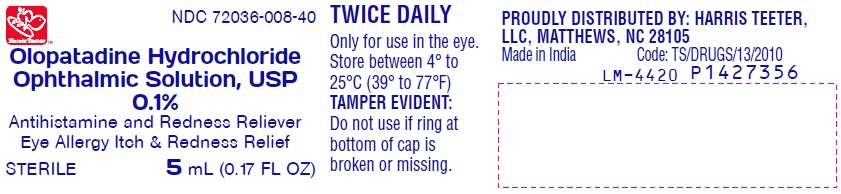
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.1% (5 mL Container)
Harris Teeter NDC 72036-008-40
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
STERILE 5 mL (0.17 FL OZ)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-0.1% (5 mL Container Carton)
NDC 72036-008-40
Harris TeeterTM
NOW AVAILABLE without a prescription
Olopatadine Hydrochloride
Ophthalmic Solution, USP
0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
Works in Minutes
Relief from Allergens:
⦁ Pet Dander ⦁ Pollen ⦁ Grass ⦁ Ragweed
*Compare to the Active Ingredient
in Pataday® Twice Daily Relief
TWICE STERILE
DAILY 5 mL (0.17 FL OZ)
