FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
These highlights do not include all the information needed to use VANCOMYCIN HYDROCHLORIDE FOR INJECTION safely and effectively. See full prescribing information for VANCOMYCIN HYDROCHLORIDE FOR INJECTION.VANCOMYCIN HYDROCHLORIDE for injection, for intravenous use
Initial U.S. Approval: 1958
1.1 Septicemia
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of septicemia due to:
- Susceptible isolates of methicillin-resistant Staphylococcus aureus (MRSA) and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.2 Infective Endocarditis
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of infective endocarditis due to:
- Susceptible isolates of MRSA.
- Viridans group streptococci Streptococcus gallolyticus (previously known as Streptococcus bovis), Enterococcus species and Corynebacterium species. For enterococcal endocarditis, use vancomycin hydrochloride for injection in combination with an aminoglycoside.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of early-onset prosthetic valve endocarditis caused by Staphylococcus epidermidis in combination with rifampin and an aminoglycoside.
1.3 Skin and Skin Structure Infections
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of skin and skin structure infections due to:
- Susceptible isolates of MRSA and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.4 Bone Infections
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of bone infections due to:
- Susceptible isolates of MRSA and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.5 Lower Respiratory Tract Infections
Vancomycin hydrochloride for injection is indicated in adults and pediatric patients (neonates and older) for the treatment of lower respiratory tract infections due to:
- Susceptible isolates of MRSA
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.6 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin hydrochloride for injection and other antibacterial drugs, vancomycin hydrochloride for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
To reduce the risk of infusion related adverse reactions, administer vancomycin hydrochloride for injection in a diluted solution over 60 minutes or greater [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Vancomycin hydrochloride for injection concentrations of no more than 5 mg/mL are recommended in adults [see Dosage and Administration (2.2)]. See also age- specific recommendations [see Dosage and Administration (2.3)]. In selected patients in need of fluid restriction, a concentration up to 10 mg/mL may be used [see Warnings and Precautions (5.1)].
Administer vancomycin hydrochloride for injection prior to intravenous anesthetic agents to reduce the risk of infusion related adverse reactions [see Warnings and Precautions (5.1)].
Administer vancomycin hydrochloride by a secure intravenous route of administration to avoid local irritation and phlebitis reactions [see Warnings and Precautions (5.8)].
The supplied lyophilized powder must be reconstituted and subsequently diluted prior to intravenous use [see Dosage and Administration (2.5)].
2.2 Dosage in Adult Patients With Normal Renal Function
The usual daily intravenous dose is 2 grams divided either as 500 mg every 6 hours or 1 g every 12 hours. Administer each dose over a period of 60 minutes or greater. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose.
2.3 Dosage in Pediatric Patients With Normal Renal Function
Pediatric Patients (Aged 1 month and older)
The usual intravenous dosage of vancomycin is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes. Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
Neonates (Up to 1 month old)
In pediatric patients, up to the age of 1 month, the total daily intravenous dosage may be lower. In neonates, an initial dose of 15 mg/kg is suggested, followed by 10 mg/kg every 12 hours for neonates in the 1st week of life and every 8 hours thereafter up to the age of 1 month. Each dose should be administered over 60 minutes. In premature infants, vancomycin clearance decreases as postconceptional age decreases. Therefore, longer dosing intervals may be necessary in premature infants. Close monitoring of serum concentrations of vancomycin is recommended in these patients.
2.4 Dosage in Patients With Renal Impairment
Dosage adjustment must be made in patients with renal impairment. The initial dose should be no less than 15 mg/kg, in patients with any degree of renal impairment.
In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Measure trough vancomycin serum concentrations to guide therapy, especially in seriously ill patients with changing renal function.
For functionally anephric patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentration. A dose of 1.9 mg/kg/24 hr should be given after the initial dose of 15 mg/kg.
2.5 Preparation of Vancomycin Hydrochloride for Injection for Intravenous Administration and Storage Instructions
Vancomycin hydrochloride for injection must be reconstituted and further diluted.
Reconstitution of the Lyophilized Powder and further dilution
At the time of use, reconstitute the vials of vancomycin hydrochloride for injection (lyophilized powder) with Sterile Water for Injection to a concentration of 50 mg of vancomycin/mL then further dilute with an infusion solution to a final concentration of 5 mg/mL (see Table 1 for the appropriate volumes). Discard any reconstituted solution remaining in the vial.
Table 1 Volume of Sterile Water for Injection to be Added for Reconstitution and Volume of Infusion Solution to be Used for Further Dilution
|
Vancomycin Strength per Vial |
Volume of Sterile Water for Injection for reconstitutiona |
Volume of infusion solutionb to further dilute to a final concentration of 5 mg/mL |
|
1.25 g |
25 mL |
250 mL |
|
1.5 g |
30 mL |
300 mL |
aAfter reconstitution, the vials may be stored in a refrigerator for 14 days without significant loss of potency.
bUse an infusion solution from the list of the compatible infusion solutions below [see Dosage and Administration (2.6)].
The desired dose diluted in this manner should be administered by intermittent IV infusion over a period of 60 minutes or greater.
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard reconstituted and diluted solutions 14 days after initial reconstitution.
2.6 Compatibility with Intravenous Fluids
The following diluents are physically and chemically compatible with 5 g/L vancomycin hydrochloride:
5% Dextrose Injection, USP
5% Dextrose Injection and
0.9% Sodium Chloride Injection, USP
Lactated Ringer’s Injection, USP
Lactated Ringer’s and 5% Dextrose Injection, USP
0.9% Sodium Chloride Injection, USP
Storage of Diluted Solutions:
Solutions that are diluted with 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP may be stored in a refrigerator for 14 days without significant loss of potency.
Solutions that are diluted with the following infusion fluids may be stored in a refrigerator for 96 hours:
5% Dextrose Injection and 0.9% Sodium Chloride Injection, USP
Lactated Ringer’s Injection, USP
Lactated Ringer’s and 5% Dextrose Injection, USP
2.7 Incompatibilities for Intravenous Use
Vancomycin solution has a low pH and may cause chemical or physical instability when it is mixed with other compounds.
Mixtures of solutions of vancomycin and beta-lactam antibacterial drugs have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between the administration of these antibacterial drugs. It is also recommended to dilute solutions of vancomycin to 5 mg/mL or less.
3 DOSAGE FORMS AND STRENGTHS
Vancomycin Hydrochloride for Injection, USP is a sterile lyophilized powder for injection supplied as an off-white to light tan colored plug or powder in single-dose vials containing vancomycin hydrochloride, USP equivalent to 1.25 g, or 1.5 g of vancomycin base.
4 CONTRAINDICATIONS
Vancomycin hydrochloride for injection is contraindicated in patients with known hypersensitivity to vancomycin.
5 WARNINGS AND PRECAUTIONS
5.1 Infusion Reactions
Hypotension, including shock and cardiac arrest, wheezing, dyspnea, urticaria, muscular and chest pain may occur with rapid vancomycin hydrochloride for injection administration. The reactions may be more severe in younger patients, particularly children, and in patients receiving concomitant muscle relaxant anesthetics.
Rapid intravenous administration of vancomycin hydrochloride for injection may also be associated with “vancomycin infusion reaction”, which manifests as pruritus and erythema that involves the face, neck and upper torso.
Infusion-related adverse reactions are related to both the concentration and the rate of administration of vancomycin. Infusion-related adverse reactions may occur, however, at any rate or concentration.
Administer vancomycin hydrochloride for injection in a diluted solution over a period of 60 minutes or greater to reduce the risk of infusion-related adverse reactions. In selected patients in need of fluid restriction, a concentration up to 10 mg/mL may be used; use of such higher concentrations may increase the risk of infusion-related adverse reactions. Administer prior to intravenous anesthetic agents when feasible. Stop the infusion if a reaction occurs.
5.2 Nephrotoxicity
Vancomycin hydrochloride for injection can result in acute kidney injury (AKI), including acute renal failure, mainly due to interstitial nephritis or less commonly acute tubular necrosis. AKI is manifested by increasing blood urea nitrogen (BUN) and serum creatinine (Cr). The risk of AKI increases with higher vancomycin serum levels, prolonged exposure, concomitant administration of other nephrotoxic drugs, concomitant administration of piperacillin-tazobactam [see Drug Interactions (7.2)], volume depletion, pre-existing renal impairment and in critically ill patients and patients with co-morbid conditions that predispose to renal impairment.
Monitor serum vancomycin concentrations and renal function in all patients receiving vancomycin hydrochloride for injection. More frequent monitoring is recommended in patients with comorbidities that predispose to impairment in renal function or are concomitantly receiving other nephrotoxic drugs, in critically ill patients, in patients with changing renal function, and in patients requiring higher therapeutic vancomycin levels. If acute kidney injury occurs, discontinue vancomycin hydrochloride for injection or reduce the dose.
5.3 Ototoxicity
Ototoxicity has occurred in patients receiving vancomycin hydrochloride for injection. It may be transient or permanent. Ototoxicity manifests as tinnitus, hearing loss, dizziness or vertigo. The risk is higher in older patients, patients who are receiving higher doses, who have an underlying hearing loss, who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside or who have underlying renal impairment. Monitor for signs and symptoms of ototoxicity during therapy. Monitor serum vancomycin concentrations and renal function in all patients receiving parenteral vancomycin. Discontinue vancomycin hydrochloride for injection if ototoxicity occurs. Dosage of vancomycin hydrochloride for injection must be adjusted for patients with renal impairment [see Dosage and Administration (2.3)]. Serial tests of auditory function may be helpful in order to minimize the risk of ototoxicity.
5.4 Severe Dermatologic Reactions
Severe dermatologic reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported in association with the use of vancomycin. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters.
Discontinue vancomycin hydrochloride for injection at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD.
5.5 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including vancomycin hydrochloride for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin.
Prolonged use of vancomycin hydrochloride for injection may result in the overgrowth of nonsusceptible microorganisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis due to C. difficile developing in patients who received intravenous vancomycin hydrochloride for injection.
5.6 Hemorrhagic Occlusive Retinal Vasculitis (HORV)
Hemorrhagic occlusive retinal vasculitis, including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established by adequate and well-controlled trials. Vancomycin is not indicated for the prophylaxis of endophthalmitis.
5.7 Neutropenia
Reversible neutropenia has been reported in patients receiving vancomycin hydrochloride for injection [see Adverse Reactions (6.1)]. Patients who will undergo prolonged therapy with vancomycin hydrochloride for injection or those who are receiving concomitant drugs which may cause neutropenia should have periodic monitoring of the leukocyte count.
5.8 Phlebitis and Other Administration Site Reactions
Inflammation at the site of injection of vancomycin hydrochloride for injection has been reported. Vancomycin hydrochloride for injection is irritating to tissue and must be given by a secure intravenous route of administration to reduce the risk of local irritation and phlebitis.
Administration of vancomycin hydrochloride for injection by intramuscular (IM), intraperitoneal, intrathecal (intralumbar or intraventricular), or intravitreal routes has not been approved and is not recommended. The safety and efficacy of vancomycin administered by the intrathecal (intralumbar or intraventricular) route or by the intraperitoneal route have not been established by adequate and well controlled trials.
Pain, tenderness, and necrosis occur with IM injection of vancomycin hydrochloride for injection or with inadvertent extravasation. Thrombophlebitis may occur, the frequency and severity of which can be minimized by administering the drug slowly as a dilute solution (2.5 to 5 g/L) and by rotation of venous access sites.
Intraperitoneal administration during continuous ambulatory peritoneal dialysis (CAPD) can result in chemical peritonitis. Manifestations range from cloudy dialysate alone to a cloudy dialysate accompanied by variable degrees of abdominal pain and fever. This syndrome appears to be resolve after discontinuation of intraperitoneal vancomycin.
5.9 Development of Drug-Resistant Bacteria
Prescribing vancomycin hydrochloride for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infusion Reactions [see Warnings and Precautions (5.1)]
- Nephrotoxicity [see Warnings and Precautions (5.2)]
- Ototoxicity [see Warnings and Precautions (5.3)]
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.5)]
- Hemorrhagic Occlusive Retinal Vasculitis [see Warnings and Precautions (5.6)]
- Neutropenia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions associated with the use of vancomycin hydrochloride for injection were identified in clinical trials:
Immune system disorders: Hypersensitivity reactions including anaphylaxis and “vancomycin infusion reaction” [see Warnings and Precautions (5.1)]
Skin and subcutaneous tissue disorders: Erythema (especially of the face, neck and upper torso) and pruritus which are manifestations of rashes including exfoliative dermatitis, toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), Linear IgA bullous dermatosis (LABD) [see Warnings and Precautions (5.4)].
Renal and urinary disorders: Acute kidney injury and interstitial nephritis
Ear and Labyrinth Disorders: Tinnitus, hearing loss, vertigo
Blood and Lymphatic System Disorders: Agranulocytosis, neutropenia, pancytopenia, leukopenia, thrombocytopenia, eosinophilia
Gastrointestinal Disorders: Pseudomembranous colitis [see Warnings and Precautions (5.5)]
Cardiac Disorders: Cardiac arrest, chest pain
General Disorders and Administration Site Conditions: General discomfort, fever, chills, phlebitis, injection site irritation, injection site pain and necrosis following intramuscular injection, chemical peritonitis following intraperitoneal administration
(Vancomycin hydrochloride for injection is not approved for intramuscular and intraperitoneal administration) [see Warnings and Precautions (5.7)]
Laboratory Abnormalities: Elevated blood urea nitrogen, elevated serum creatinine
Musculoskeletal and connective tissue disorders: Muscle pain
Nervous system disorders: Dizziness
Respiratory, thoracic and mediastinal disorders: Wheezing, dyspnea
Vascular disorders: Hypotension, shock, vasculitis
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of vancomycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP) [see Warnings and Precautions (5.4)].
7 DRUG INTERACTIONS
Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
7.1 Anesthetic Agents
Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
7.2 Piperacillin-Tazobactam
Studies have detected an increased incidence of acute kidney injury in patients administered concomitant piperacillin/tazobactam and vancomycin as compared to vancomycin alone. Monitor kidney function in patients receiving concomitant piperacillin/tazobactam and vancomycin. No pharmacokinetic interactions have been noted between piperacillin/tazobactam and vancomycin.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on vancomycin use in pregnant women to inform a drug associated risk of major birth defects or miscarriage. Available published data on vancomycin use in pregnancy during the second and third trimesters have not shown an association with adverse pregnancy related outcomes (see Data). Vancomycin did not show adverse developmental effects when administered intravenously to pregnant rats and rabbits during organogenesis at doses less than or equal to the recommended maximum human dose based on body surface area (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
A published study evaluated hearing loss and nephrotoxicity in infants of pregnant intravenous drug users treated with vancomycin for suspected or documented methicillin-resistant Staphylococcus-aureus in the second or third trimester. The comparison groups were 10 non-intravenous drug-dependent patients who received no treatment, and 10 untreated intravenous drug-dependent patients who served as substance abuse controls. No infant in the vancomycin exposed group had abnormal sensorineural hearing at 3 months of age or nephrotoxicity.
A published prospective study assessed outcomes in 55 pregnant women with a positive Group B streptococcus (GBS) culture and a high-risk penicillin allergy with resistance to clindamycin or unknown sensitivity who were administered vancomycin at the time of delivery. Vancomycin dosing ranged from the standard 1 g intravenously every 12 hours to 20 mg/kg intravenous every 8 hours (maximum individual dose 2 g). No major adverse reactions were recorded either in the mothers or their newborns. None of the newborns had sensorineural hearing loss. Neonatal renal function was not examined, but all of the newborns were discharged in good condition.
Animal Data
Vancomycin did not cause fetal malformations when administered during organogenesis to pregnant rats (gestation days 6 to 15) and rabbits (gestation days 6 to 18) at the equivalent recommended maximum human dose (based on body surface area comparisons) of 200 mg/kg/day IV to rats or 120 mg/kg/day IV to rabbits. No effects on fetal weight or development were seen in rats at the highest dose tested or in rabbits given 80 mg/kg/day (approximately 1 and 0.8 times the recommended maximum human dose based on body surface area, respectively). Maternal toxicity was observed in rats (at doses 120 mg/kg and above) and rabbits (at 80 mg/kg and above).
8.2 Lactation
Risk Summary
There are insufficient data to inform the levels of vancomycin in human milk. There are no data on the effects of vancomycin on the breastfed infant or milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for vancomycin and any potential adverse effects on the breastfed infant from vancomycin or from the underlying maternal condition.
8.4 Pediatric Use
Vancomycin hydrochloride for injection is indicated in pediatric patients (neonates and older). In pediatric patients, monitor vancomycin serum concentration and renal function when administering vancomycin hydrochloride for injection [see Dosage and Administration (2.2, 2.3) and Warnings and Precautions (5.2)]. More severe infusion related reactions related to vancomycin administration may occur in pediatric patients. Concomitant administration of vancomycin and intravenous anesthetic agents has been associated with erythema and histamine-like flushing in all patients including pediatric patients [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Vancomycin hydrochloride for injection is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection [see Dosage and Administration (2.2)], and it may be useful to monitor renal function [see Warnings and Precautions (5.2)].
10 OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance.
For current information on the management of overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
11 DESCRIPTION
Vancomycin Hydrochloride for Injection, USP, contains the hydrochloride salt of vancomycin, a tricyclic glycopeptide antibacterial derived from Amycolatopsis orientalis (formerly Nocardia orientalis). The chemical name for vancomycin hydrochloride, USP is (Sa)- (3S,6R,7R,22R,23S,26S,36R,38aR)-44-[[2-O-(3-Amino-2,3,6-trideoxy-3-C-methyl-α-L-lyxo-hexopyranosyl)-β-D-glucopyranosyl]oxy]-3-(carbamoylmethyl)-10,19-dichloro- 2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[(2R)-4- methyl-2-(methylamino)valeramido]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36- (iminomethano)-13,16:31,35-dimetheno-1H,16H-[l,6,9]oxadiazacyclohexadecino[4,5- m][10,2,16]-benzoxadiazacyclotetracosine-26-carboxylic acid, monohydrochloride. The molecular formula is C66H75Cl2N9O24 • HCl and the molecular weight is 1,485.71. Vancomycin hydrochloride, USP has the following structural formula:
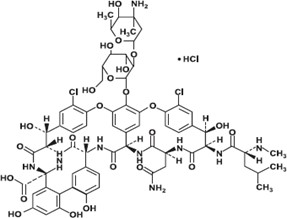
Vancomycin Hydrochloride for Injection, USP is a sterile off-white to light tan colored lyophilized plug or powder for injection. Vancomycin Hydrochloride for Injection, USP is supplied in single- dose vials, containing, 1.28 g, or 1.54 g of vancomycin hydrochloride, USP equivalent to, 1.25 g, or 1.5 g of vancomycin base. The lyophilized powder is reconstituted with Sterile Water for Injection, USP which forms a clear, colorless or light to dark tan solution and subsequently diluted prior to intravenous administration [see Dosage and Administration (2.5)].
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
In subjects with normal kidney function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63 mcg/mL immediately after the completion of infusion, mean plasma concentrations of approximately 23 mcg/mL 2 hours after infusion, and mean plasma concentrations of approximately 8 mcg/mL 11 hours after the end of the infusion. Multiple dosing of 500 mg infused over 30 minutes produces mean plasma concentrations of about 49 mcg/mL at the completion of infusion, mean plasma concentrations of about 19 mcg/mL 2 hours after infusion, and mean plasma concentrations of about 10 mcg/mL 6 hours after infusion. The plasma concentrations during multiple dosing are like those after a single dose.
Distribution
The volume of distribution ranges from 0.3 to 0.43 L/kg after intravenous administration. Vancomycin is approximately 55% serum protein bound as measured by ultrafiltration at vancomycin serum concentrations of 10 to 100 mcg/mL. After intravenous administration of vancomycin, inhibitory concentrations are present in pleural, pericardial, ascitic, and synovial fluids; in urine; in peritoneal dialysis fluid; and in atrial appendage tissue. Vancomycin does not readily diffuse across normal meninges into the spinal fluid; but, when the meninges are inflamed, penetration into the spinal fluid occurs.
Elimination
Mean plasma clearance is about 0.058 L/kg/h, and mean renal clearance is about 0.048 L/kg/h. The mean elimination half-life of vancomycin from plasma is 4 to 6 hours in subjects with normal renal function. In anephric patients, the mean elimination half-life is 7.5 days. Total body and renal clearance of vancomycin may be reduced in the elderly.
Metabolism
There is no apparent metabolism of the vancomycin.
Excretion
In the first 24 hours after intravenous administration, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration. Renal impairment slows excretion of vancomycin.
About 60% of an intraperitoneal dose of vancomycin administered during peritoneal dialysis is absorbed systemically in 6 hours. Serum concentrations of about 10 mcg/mL are achieved by intraperitoneal injection of 30 mg/kg of vancomycin. However, the safety and efficacy of the intraperitoneal use of vancomycin has not been established in adequate and well-controlled trials [see Warnings and Precautions (5.7)].
12.4 Microbiology
Mechanism of Action
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis.
Resistance
Vancomycin is not active in vitro against gram-negative bacilli, mycobacteria, or fungi. There is no cross-resistance between vancomycin and other antibacterials.
Interaction With Other Antimicrobials
The combination of vancomycin and an aminoglycoside acts synergistically in vitro against many isolates of Staphylococcus aureus, Streptococcus gallolyticus (previously known as Streptococcus bovis), enterococcus spp, and the viridans group streptococci.
Antimicrobial Activity
Vancomycin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Aerobic Gram-Positive Bacteria
Corynebacterium spp.
Enterococcus spp. (including Enterococcus faecalis)
Staphylococcus aureus (including methicillin-resistant and methicillin-susceptible isolates)
Coagulase negative staphylococci (including S. epidermidis and methicillin-resistant isolates)
Streptococcus gallolyticus (previously known as Streptococcus bovis)
Viridans group streptococci
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for vancomycin against isolates of similar genus or organism group. However, the efficacy of vancomycin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic Gram-Positive Bacteria
Listeria monocytogenes
Streptococcus pyogenes
Streptococcus pneumoniae
Streptococcus agalactiae
Anaerobic Gram-Positive Bacteria
Actinomyces species
Lactobacillus species
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no mutagenic potential of vancomycin hydrochloride for injection was found in standard laboratory tests. No definitive fertility studies have been performed.
15 REFERENCES
1. Byrd RA., Gries CL, Buening M.: Developmental Toxicology Studies of Vancomycin Hydrochloride Administered Intravenously to Rats and Rabbits. Fundam Appl Toxicol 1994; 23: 590-597.
16 HOW SUPPLIED/STORAGE AND HANDELING
How Supplied:
Vancomycin Hydrochloride for Injection, USP is a sterile lyophilized powder for injection supplied as an off-white to light tan colored powder or plug in single-dose flip top vials that contain vancomycin hydrochloride, USP equivalent to 1.25 g, or 1.5 g of vancomycin base. They are available as follows:
NDC 0143-9152-10: Vancomycin Hydrochloride for Injection, USP equivalent to 1.25 g vancomycin in a 50 mL flip top vial with a light violet seal, in packages of 10 vials.
NDC 0143-9153-10: Vancomycin Hydrochloride for Injection, USP equivalent to 1.5 g vancomycin in a 50 mL flip top vial with a white seal, in packages of 10 vials.
Storage:
Prior to reconstitution, store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.]
17 PATIENT COUNSELING INFORMATION
Infusion Reactions During or After Intravenous Use
Advise patients that generalized skin redness, skin rash, itching, flushing, muscle pain, chest pain, shortness of breath, wheezing, or dizziness may occur during intravenous infusion of vancomycin hydrochloride for injection. These reactions can be lessened or prevented by infusing the drug over at least 60 minutes [see Warnings and Precautions (5.1)].
Acute Kidney Injury
Advise patients that vancomycin hydrochloride for injection can result in kidney damage and that blood tests are required to monitor vancomycin blood levels and kidney function during therapy. [see Warnings and Precautions (5.2)].
Hearing Loss or Balance Problems
Advise patients that vancomycin hydrochloride for injection may result in decreased hearing and to report hearing loss or balance problems to their healthcare provider [see Warnings and Precautions (5.3)].
Severe Dermatologic Reactions
Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop vancomycin hydrochloride for injection immediately and promptly seek medical attention at the first signs or symptoms of skin rash, mucosal lesions or blisters [see Warnings and Precautions (5.4)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including vancomycin hydrochloride for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When vancomycin hydrochloride for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Vancomycin hydrochloride for injection or other antibacterial drugs in the future.
Diarrhea
Diarrhea is a common problem caused by antibacterial drugs, including vancomycin, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible [see Warnings and Precautions (5.5)].
The brands listed are trademarks of their respective owners.
Manufactured by:
HIKMA FARMACÊUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B – Fervença – 2705-906 Terrugem SNT, PORTUGAL
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised February 2023
PIN624-WES/2
PRINCIPAL DISPLAY PANEL - 1.25 g
NDC 0143-9152-01 Rx only
Vancomycin Hydrochloride for Injection, USP
1.25 g* per vial
For Intravenous Use
AFTER RECONSTITUTION MUST BE FURTHER DILUTED.
SEE PACKAGE INSERT.
Sterile Powder
Single-Dose Vial

NDC 0143-9152-10 Rx only
Vancomycin Hydrochloride for Injection, USP
1.25 g* per vial
For Intravenous Use
AFTER RECONSTITUTION MUST BE FURTHER DILUTED.
SEE PACKAGE INSERT.
Sterile Powder
10 x 1.25 g Single-Dose Vials

PRINCIPAL DISPLAY PANEL - 1.5 g
NDC 0143-9153-01 Rx only
Vancomycin Hydrochloride for Injection, USP
1.5 g* per vial
For Intravenous Use
AFTER RECONSTITUTION MUST BE FURTHER DILUTED.
SEE PACKAGE INSERT.
Sterile Powder
Single-Dose Vial

NDC 0143-9153-10 Rx only
Vancomycin Hydrochloride for Injection, USP
1.5 g* per vial
For Intravenous Use
AFTER RECONSTITUTION MUST BE FURTHER DILUTED.
SEE PACKAGE INSERT.
Sterile Powder
10 x 1.5 g Single-Dose Vials

