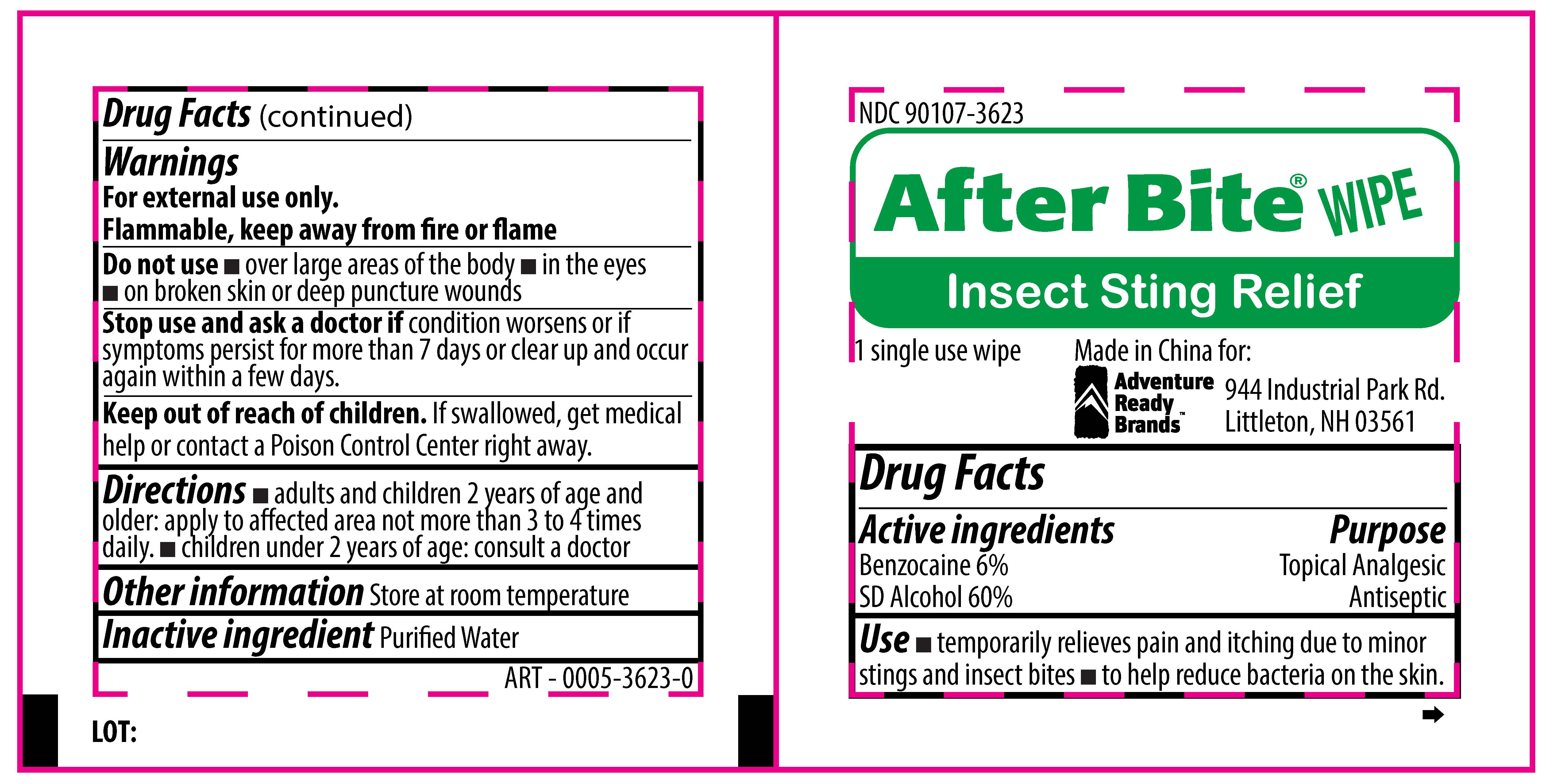
AFTER BITE WIPE INSECT STING RELIEF- benzocaine,alcohol swab
Adventure Ready Brands
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Benzocaine, 6%
SD alcohol, 60%
Purpose
Topical Analgesic
Antiseptic
Use
- temporarily relieves pain and itching due to minor stings and insect bites
- to help reduce bacteria on the skin
Warnings
For external use only.
Flammable, keep away from fire or flame.
Do not use
- over large areas of the body
- in the eyes
- on broken skin or deep puncture wounds
Stop use and ask a doctor if
condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 year of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: consult a doctor
Other information
Store at room temperature
Inactive Ingredient
Purified water