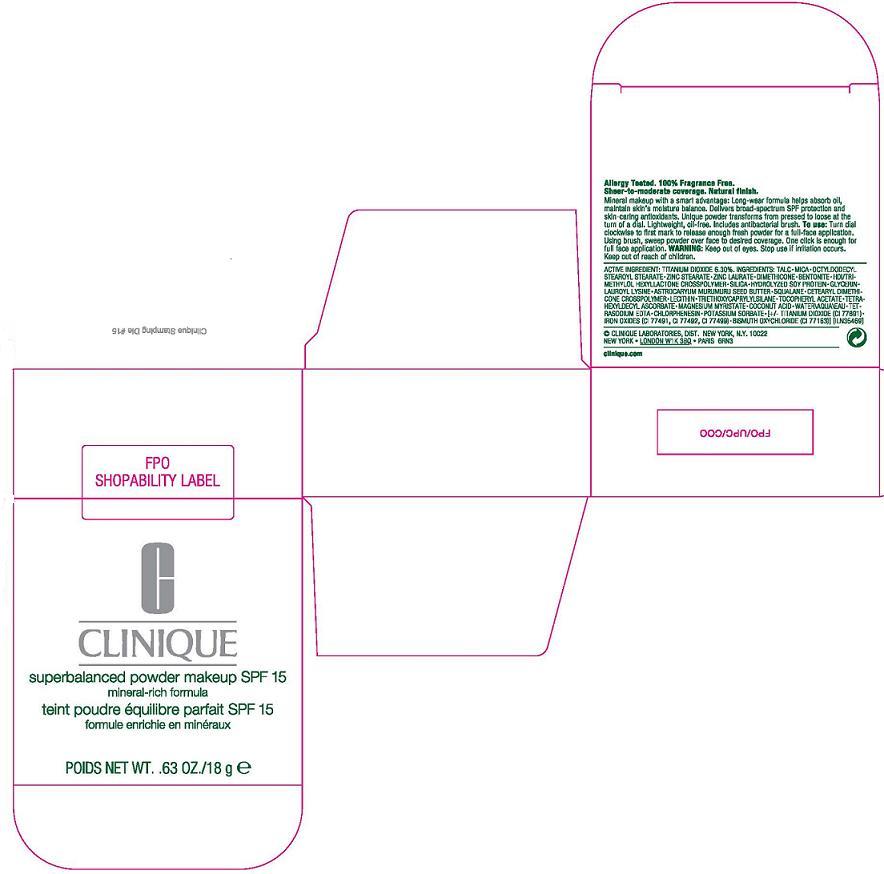
inactive ingredients: talc [] mica [] octyldodecyl stearoyl stearate [] zinc stearate [] zinc laurate [] dimethicone [] bentonite [] hdi/trimethylol hexyllactone crosspolymer [] silica [] hydrolyzed soy protein [] glycerin [] lauroyl lysine [] astrocaryum murumuru seed butter [] squalane [] cetearyl dimethicone crosspolymer [] lecithin [] triethoxycaprylylsilane [] tocopheryl acetate [] tetrahexyldecyl ascorbate [] magnesium myristate [] coconut acid [] water\aqua\eau [] tetrasodium edta [] chlorphenesin [] potassium sorbate [] [+/- titanium dioxide (ci 77891) [] iron oxides (ci 77491, ci 77492, ci 77499) [] bismuth oxychloride (ci 77163)