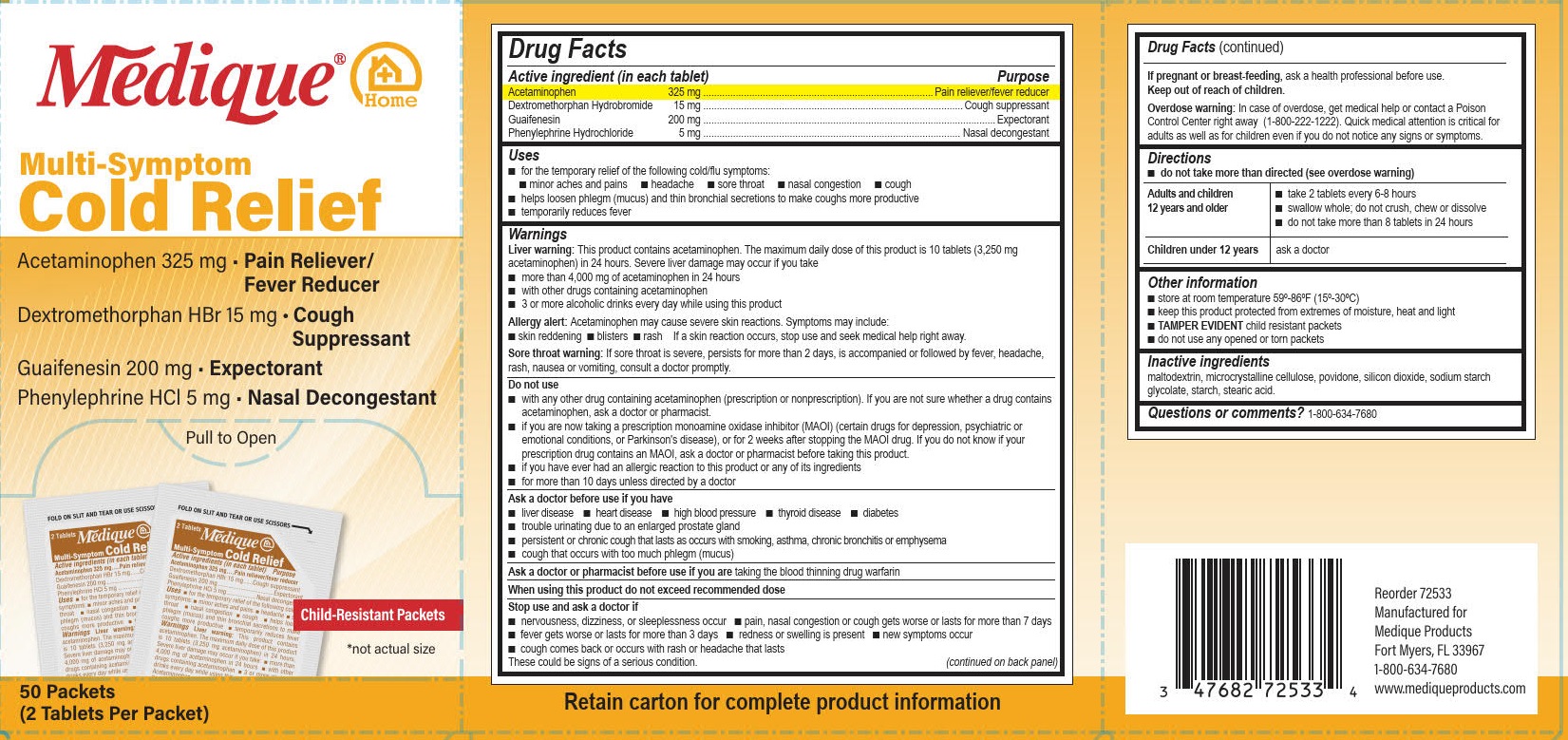
Active ingredients (in each tablet)
Acetaminophen 325 mg
Dextromethorphan Hydrobromide 15 mg
Guaifenesin 200 mg
Phenylephrine Hydrochloride 5 mg .
Uses
■ for the temporary relief of the following cold/flu symptoms:
■ minor aches and pains ■ headache ■ sore throat ■ nasal congestion ■ cough
■ helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
■ temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 10 tablets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
■ more than 4,000 mg of acetaminophen in 24 hours
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
■ skin reddening
■ blisters
■ rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a
drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression,
psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If
you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this
product.
■ if you have ever had an allergic reaction to this product or any of its ingredients
■ for more than 10 days unless directed by a doctor
Ask a doctor before use if you have
■ liver disease
■ heart disease
■ high blood pressure
■ thyroid disease
■ diabetes
■ trouble urinating due to an enlarged prostate gland
■ persistent or chronic cough that lasts as occurs with smoking, asthma, chronic bronchitis or emphysema
■ cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
■ nervousness, dizziness, or sleeplessness occur
■ pain, nasal congestion or cough gets worse or lasts for more than 7 days
■ fever gets worse or lasts for more than 3 days
■ redness or swelling is present
■ new symptoms occur
■ cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
■ do not take more than directed (see overdose warning)
Adults and children12 years and older
■ take 2 tablets every 6-8 hours
■ swallow whole; do not crush, chew or dissolve
■ do not take more than 8 tablets in 24 hours
Children under 12 years
ask a doctor
Other information
■ store at room temperature 68º-77ºF (20º-25ºC)
■ keep this product protected from extremes of moisture, heat and light
■ Tamper Evident child resistant packets
■ do not use any opened or torn packets
Inactive ingredients
maltodextrin, microcrystalline cellulose, povidone, silicon dioxide, sodium starch glycolate, starch, stearic acid
Medique® @ Home
Multi-Symptom Cold Relief
Acetaminophen 325 mg • Pain Reliever/ Fever Reducer
Dextromethorphan Hydrobromide 15 mg • Cough Suppressant
Guaifenesin 200 mg • Expectorant
Phenylephrine Hydrochloride 5 mg • Nasal Decongestant
Pull to Open
Child-Resistant Packets
*not actual size
50 Packets
(2 Tablets Per Packet)