Uses
- ▪
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- ▪
- temporarily relieves:
- ▪
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- ▪
- the intensity of coughing
- ▪
- the impulse to cough to help your child get to sleep
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- ▪
- cough that occurs with too much phlegm (mucus)
- ▪
- persistent or chronic cough such as occurs with asthma
Directions
- ▪
- do not take more than 6 doses in any 24-hour period
- ▪
- measure only with dosing cup provided
- ▪
- do not use dosing cup with other products
- ▪
- mL = milliliter
| Age | Dose |
|---|---|
|
children 6 years to under 12 years |
5 mL – 10 mL every 4 hours |
|
children 4 years to under 6 years |
2.5 mL – 5 mL every 4 hours |
|
children under 4 years |
do not use |
Other information
- ▪
- each 5 mL contains: sodium 3 mg
- ▪
- Tamper evident: do not use if printed inner seal under cap is torn or missing
- ▪
- store at room temperature
- ▪
- do not refrigerate
- ▪
- dosing cup provided
Inactive ingredients
anhydrous citric acid, edetate disodium, FD&C red #40, flavors, potassium citrate, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum
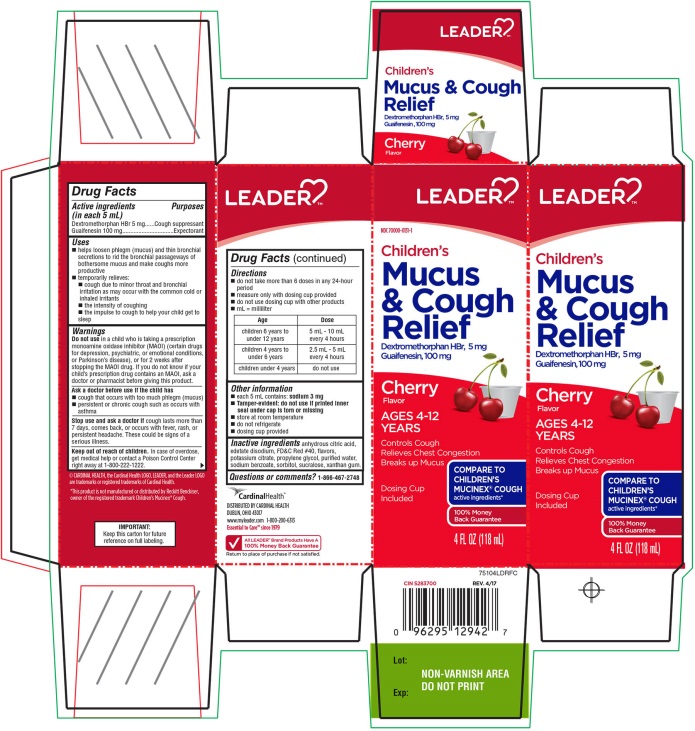
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
LEADER™
NDC 70000-0131-1
Children's Mucus and Cough Relief
Dextromethorphan HBr , 5 mg
Guaifenesin, 100 mg
Cherry
Flavor
AGES 4-12 YEARS
Controls Cough
Relieves Chest Congestion
Breaks up Mucus
COMPARE TO CHILDREN’S MUCINEX® COUGH active ingredients
100% Money Back Guarantee
Dosing Cup included
4 FL OZ
(118 mL)
- © CARDINAL HEALTH, the Cardinal Health LOGO, LEADER, and the Leader LOGO are trademarks or registered trademarks of Cardinal Health.
*This product is not manufactured or distributed by Reckitt Benckiser, owner of the registered trademark Children’s Mucinex® Cough.
|
IMPORTANT: Keep this carton for future reference on full labeling. |
- CARDINAL HEALTH®
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
1-800-200-6313
Essential to Care™ since 1979
All Leader Brand Products have a 100% Money Back Guarantee
Return to place of purchase if not satisfied