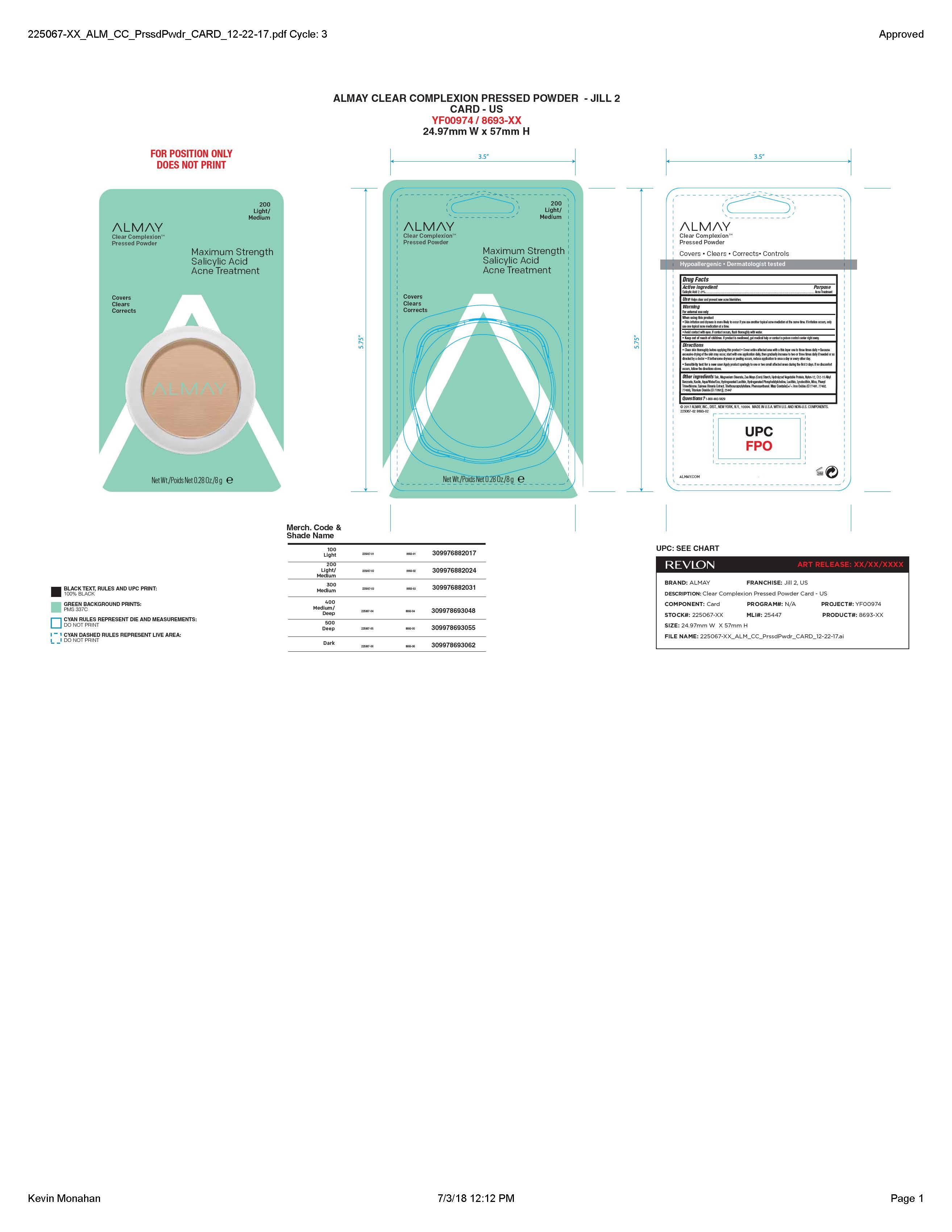
Warnings:
For external use only.
Skin irritation and dryness is likely to occur if you use antother topica; acne medication at the same time. If irritation occurs, use only one topical acne medication at a time.
Avoid contact with eyes. If contact occurs, flush throughly with water.
Directions
Clean skin thoroughly
before applying this product • Cover the
entire affected area with a thin layer 1 to 3
times daily. • Because excessive drying of
the skin may occur, start with 1 appli -
cation daily, then gradually increase to 2
or 3 times daily if needed or as directed
by doctor. • If bothersome dryness or
peeling occurs, reduce application to once
a day or every other day.
Inactive Ingredients
TALC, MAGNESIUM STEARATE, ZEA MAYS (CORN) STARCH, C12-15 ALKYL BENZOATE, NYLON-12, KAOLIN, PHENYL TRIMETHICONE, PHENOXYETHANOL, HYDROLYZED VEGETABLE PROTEIN, HYDROGENATED LECITHIN, SPIRAEA ULMARIA EXTRACT, METHYLSALICYLATYL GLYCYRRHETINATE, SILICA, SODIUM HYALURONATE