DESCRIPTION
Silver sulfadiazine cream, USP 1% is a soft, white, water dispersible cream containing the antimicrobial agent silver sulfadiazine in micronized form for topical application. Each gram of silver sulfadiazine cream contains 10mg of micronized silver sulfadiazine.
This active agent has the following structural formula:
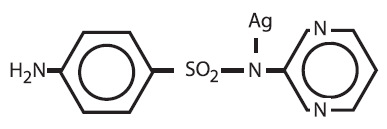
Silver sulfadiazine cream contains 1% w/w silver sulfadiazine. The vehicle in which the active ingredient is dispersed consists of water, stearyl alcohol, white petrolatum, polyoxyl 40 stearate, propylene glycol, isopropyl myristate, and sorbitan monooleate with 0.3% methylparaben as a preservative.
CLINICAL PHARMACOLOGY
Silver sulfadiazine has broad antimicrobial activity. It is bactericidal for many gram-negative and gram-positive bacteria as well as being effective against yeast. Results from in vitro testing are listed below.
Sufficient data have been obtained to demonstrate that silver sulfadiazine will inhibit bacteria that are resistant to other antimicrobial agents and that the compound is superior to sulfadiazine.
Studies utilizing radioactive micronized silver sulfadiazine, electron microscopy, and biochemical techniques have revealed that the mechanism of action of silver sulfadiazine on bacteria differs from silver nitrate and sodium sulfadiazine. Silver sulfadiazine acts only on the cell membrane and cell wall to produce its bactericidal effect.
| Results of in Vitro Testing With Silver Sulfadiazine Cream, USP 1% Concentration of Silver Sulfadiazine Number of Sensitive Strains / Total Number of Strains Tested |
||
| Genus and Species | 50 µg/mL | 100 µg/mL |
| Pseudomonas aeruginosa | 130/130 | 130/130 |
| Xanthomonas (Pseudomonas) Maltophilia | 7/7 | 7/7 |
| Enterobacter species | 48/50 | 50/50 |
| Enterobacter cloacae | 24/24 | 24/24 |
| Klebsiella species | 53/54 | 54/54 |
| Escherichia coli | 63/63 | 63/63 |
| Serratia species | 27/28 | 28/28 |
| Proteus mirabilis | 53/53 | 53/53 |
| Morganella morganii | 10/10 | 10/10 |
| Providencia rettgeri | 2/2 | 2/2 |
| Proteus vulgaris | 2/2 | 2/2 |
| Providencia species | 1/1 | 1/1 |
| Citrobacter species | 10/10 | 10/10 |
| Acinetobacter calcoaceticus | 10/11 | 11/11 |
| Stahylococcus aureus | 100/101 | 100/101 |
| Staphylococcus epidermidis | 51/51 | 51/51 |
| β-Hemolytic Streptococcus | 4/4 | 4/4 |
| Enterococcus species | 52/53 | 52/53 |
| Corynebacterium diphtheriae | 2/2 | 2/2 |
| Clostridium perfringens | 0/2 | 2/2 |
| Candida albicans | 43/50 | 50/50 |
Silver sulfadiazine is not a carbonic anhydrase inhibitor and may be useful in situations where such agents are contraindicated.
INDICATIONS AND USAGE
Silver sulfadiazine cream, USP 1% is a topical antimicrobial drug indicated as an adjunct for the prevention and treatment of wound sepsis in patients with second and third degree burns.
CONTRAINDICATIONS
Silver sulfadiazine cream, USP 1% is contraindicated in patients who are hypersensitive to silver sulfadiazine or any of the other ingredients in the preparation.
Because sulfonamide therapy is known to increase the possibility of kernicterus, silver sulfadiazine cream, USP 1% should not be used on pregnant women approaching or at term, on premature infants, or on newborn infants during the first 2 months of life.
WARNINGS
Absorption of silver sulfadiazine varies depending upon the percent of body surface area and the extent of the tissue damage. Although few have been reported, it is possible that any adverse reaction associated with sulfonamides may occur. Some of the reactions which have been associated with sulfonamides are as follows: blood dyscrasias including agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, and hemolytic anemia; dermatologic and allergic reactions, including life-threatening cutaneous reactions [Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and exfoliative dermatitis]; gastrointestinal reactions, hepatitis and hepatocellular necrosis; CNS reactions; and toxic nephrosis.
There is a potential cross-sensitivity between silver sulfadiazine and other sulfonamides. If allergic reactions attributable to treatment with silver sulfadiazine occur, continuation of therapy must be weighed against the potential hazards of the particular allergic reaction.
Fungal proliferation in and below the eschar may occur. However, the incidence of clinically reported fungal superinfection is low.
The use of silver sulfadiazine cream, USP 1% in some cases of glucose-6-phosphate dehydrogenase-deficient individuals may be hazardous, as hemolysis may occur.
PRECAUTIONS
General. If hepatic and renal functions become impaired and elimination of the drug decreases accumulation may occur. Discontinuation of silver sulfadiazine cream, USP 1% should be weighed against the therapeutic benefit being achieved.
In considering the use of topical proteolytic enzymes in conjunction with Silver sulfadiazine cream, USP 1% the possibility should be noted that silver may inactivate such enzymes.
Laboratory Tests. In the treatment of burn wounds involving extensive areas of the body, the serum sulfa concentrations may approach adult therapeutic levels (8 to 12mg %). Therefore, in these patients it would be advisable to monitor serum sulfa concentrations. Renal function should be carefully monitored and the urine should be checked for sulfa crystals.
Absorption of the propylene glycol vehicle has been reported to affect serum osmolality, which may affect the interpretation of laboratory tests.
PREGNANCY:
TERATOGENIC EFFECTS:
Pregnancy Category B. A reproductive study has been performed in rabbits at doses up to three to ten times the concentration of silver sulfadiazine in silver sulfadiazine cream, USP 1% and has revealed no evidence of harm to the fetus due to silver sulfadiazine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly justified, especially in pregnant women approaching or at term. (See CONTRAINDICATIONS)
Nursing Mothers. It is not known whether silver sulfadiazine cream, USP 1% is excreted in human milk. However, sulfonamides are known to be excreted in human milk and all sulfonamides derivatives are known to increase the possibility of kernicterus. Because of the possibility for serious adverse reactions in nursing infants from sulfonamides, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use. Of the total number of subjects in clinical studies of silver sulfadiazine cream, USP 1% seven percent were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Pediatric Use. Safety and effectiveness in children have not been established. (See CONTRAINDICATIONS)
ADVERSE REACTIONS
Several cases of transient leukopenia have been reported in patients receiving silver sulfadiazine therapy.1,2,3 Leukopenia associated with silver sulfadiazine administration is primarily characterized by decreased neutrophil count. Maximal white blood cell depression occurs within two to four days of initiation of therapy. Rebound to normal leukocyte levels follows onset within two to three days. Recovery is not influenced by continuation of silver sulfadiazine therapy. An increased incidence has been seen in patients treated concurrently with cimetidine.
Other infrequently occurring events include skin necrosis, erythema multiforme, skin discoloration, burning sensation, rashes, and interstitial nephritis.
Reduction in bacterial growth after application of topical antibacterial agents has been reported to permit spontaneous healing of deep partial-thickness burns by preventing conversion of the partial thickness to full thickness by sepsis. However, reduction in bacterial colonization has caused delayed separation, in some cases necessitating escharotomy in order to prevent contracture.
DOSAGE AND ADMINISTRATION
Prompt institution of appropriate regimens for care of the burned patient is of prime importance and includes the control of shock and pain. The burn wounds are then cleansed and debrided; silver sulfadiazine cream, USP 1% is then applied under sterile conditions. The burn areas should be covered with silver sulfadiazine cream, USP 1% at all times. The cream should be applied once to twice daily to a thickness of approximately one sixteenth of an inch. Whenever necessary, the cream should be reapplied to any areas from which it has been removed by patient activity. Administration may be accomplished in minimal time because dressings are not required. However, if individual patient requirements make dressings necessary, they may be used.
Reapply immediately after hydrotherapy. Treatment with silver sulfadiazine cream, USP 1% should be continued until satisfactory healing has occurred or until the burn site is ready for grafting. The drug should not be withdrawn from the therapeutic regimen while there remains the possibility of infection except if a significant adverse reaction occurs.
REFERENCES
- Caffee F, Bingham H. Leukopenia and silver sulfadiazine. J Trauma. 1982;22: 586–587.
- Jarret F, Ellerbe S, Demling R. Acute leukopenia during topical burn therapy with silver sulfadiazine. Amer J Surg. 1978;135: 818–819.
- Kiker RG, Carvajal HF, Micak RP, Larson DL. A controlled study of the effects of silver sulfadiazine on white blood cell counts in burned children. J Trauma. 1977;17: 835–836.
HOW SUPPLIED
| Silver sulfadiazine cream, USP 1% - white to off-white cream | ||
| NDC Number | Size |
|
| 67877-124-20 | 20g tube | |
| 67877-124-25 | 25g tube | |
| 67877-124-05 | 50g tube | |
| 67877-124-85 | 85g tube | |
| 67877-124-50 | 50g jar | |
| 67877-124-40 | 400g jar | |
| 67877-124-10 | 1000g jar | |
Store at room temperature. 15°-30°C (59°-86°F).
Manufactured for:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Manufactured by:
Crown Laboratories, Inc.
Johnson City, TN 37604
Product Information: 1-877-272-7901
Revised: DEC 2018
P1840.05
———PRINCIPAL DISPLAY PANEL———
Silver sulfadiazine (micronized) 1% in a hydrophilic (water dispersible) base consisting of water, stearyl alcohol, white petrolatum, polyoxyl 40 stearate, propylene glycol, isopropyl myristate, sorbitan monooleate, with 0.3% methylparaben as a preservative.
DOSAGE AND ADMINISTRATION: See insert provided with this package.
WARNING: KEEP OUT OF REACH OF CHILDREN.
STORE AT ROOM TEMPERATURE 15°-30°C (59° - 86°F).
For Topical Use Only. Keep tube tightly closed. See Crimp for Control Number/Expiration Date.
Mfd. for: Ascend Laboratories, LLC Parsippany, NJ 07054
Mfd. by: Crown Laboratories, Inc. Johnson City, TN 37604
Product information: 1-877-272-7901 P1837.03
NDC 67877-124-25
ASCEND Laboratories, LLC
Silver Sulfadiazine Cream, USP 1% Rx ONLY Net Wt. 25 g

